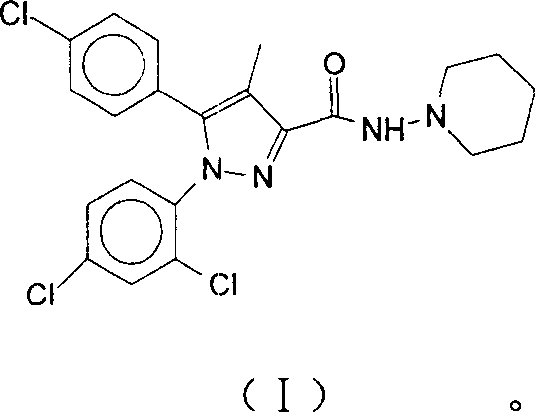
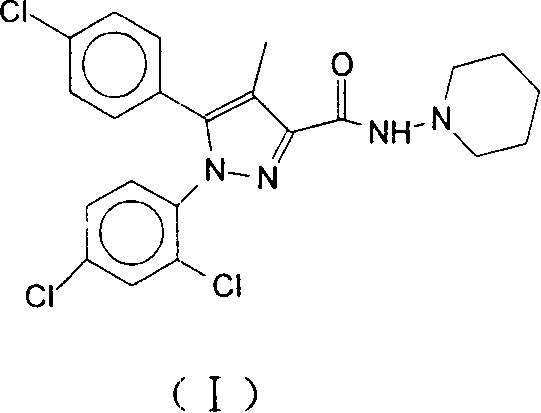
Oral cavity rapid disintegrating tablet medicament and method of preparing the same
A technology for oral fast disintegrating tablet and medicine, applied in the field of oral fast disintegrating tablet medicine and preparation thereof, can solve the problems of patients being unable to take care of themselves, inconvenient to use injections, etc., and achieve shortened drug dissolution time, good appearance and good wear resistance. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
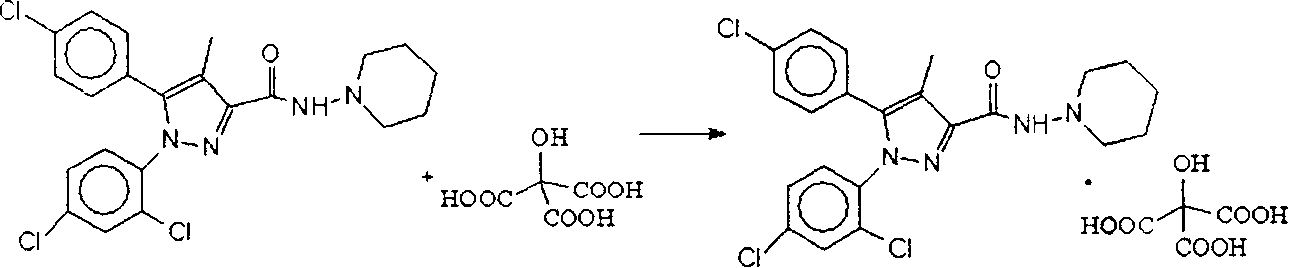
[0023] Embodiment 1 Preparation of Limoraban Citrate
[0024] Add 46.4g of rimoraban and 25.5g of anhydrous citric acid into a 500ml reaction flask, add 300ml of absolute ethanol, heat to reflux for 30min, add 2g of activated carbon, and then reflux for 30min. Filtrate while it is hot, cool the filtrate at room temperature first, and then freeze it in the refrigerator to precipitate white crystals, filter, and dry under reduced pressure to obtain 43.8 g of limoraban citrate product, with a yield of 79.7%. The reaction process is shown in the following formula:
[0025]
Embodiment 2
[0026] Example 2 Preparation of Limoraban Lactate
[0027] Add 46.4g of rimoraban and 11.5g of lactic acid into a 500ml reaction flask, add 300ml of absolute ethanol, heat to reflux for 30min, add 2g of activated carbon, and then reflux for 30min. Filtrate while it is hot, cool the filtrate to room temperature and put it in the refrigerator to freeze. White crystals are precipitated, filtered, and dried under reduced pressure to obtain 43.8 g of limoraban lactate product, with a yield of 79.7%. The reaction process is shown in the following formula:
[0028]
Embodiment 3~5
[0029] Examples 3-5 Preparation of Limoraban Citrate Orally Disintegrating Tablets
[0030] Drug A: Limoraban Citrate 5.0g
[0031] 70g microcrystalline cellulose
[0032] Low-substituted hydroxypropyl cellulose 5g
[0033] Micronized silica gel 2g
[0036] Starch 15g
[0037] Drug B: Limoraban citrate 10.0 g
[0038] 65g microcrystalline cellulose
[0039] Low-substituted hydroxypropyl cellulose 5g
[0040] Micronized silica gel 2g
[0043] Starch 15g
[0044] Drug C: Limoraban Citrate 20.0g
[0045] Microcrystalline Cellulose 55g
[0046] Low-substituted hydroxypropyl cellulose 5g
[0047] Micronized silica gel 2g
[...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com