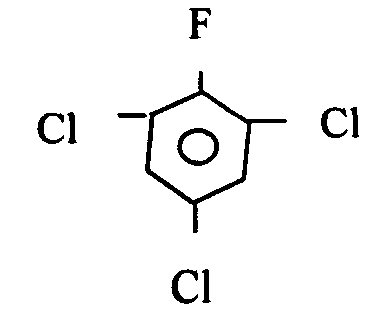
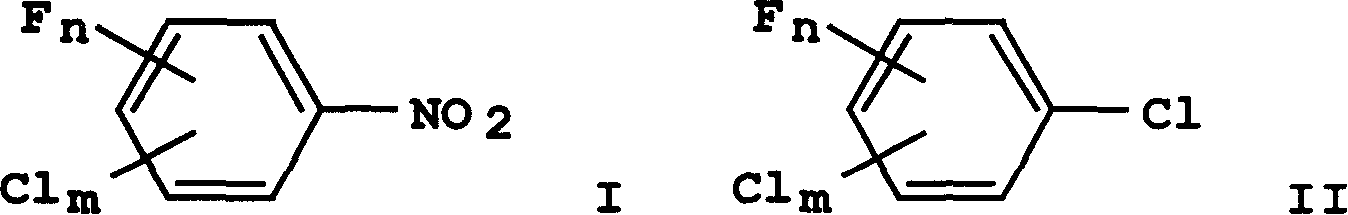Method for preparing 2,4,6-trichloro-fluorobenzene
A technology of trichlorofluorobenzene and fluoronitrobenzene, applied in 2 fields, can solve the problems of low yield, high reaction temperature, harsh equipment conditions, etc., improve the conversion rate of raw materials, save existing resources, and solve pollution and waste Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Produce high boiler (i.e. the mixture of trichloronitrobenzene) analysis when getting p-chloronitrobenzene chlorination to produce 3,4-dichloronitrobenzene:
[0033] 3,4,5-trichloronitrobenzene accounts for 70%; 2,3,4-trichloronitrobenzene accounts for 15.7%; 2,4,5-trichloronitrobenzene accounts for 15-20%
[0034] The mixed trichloronitrobenzene and potassium fluoride (KF) after destaining are reacted with the quaternary ammonium salt catalyst at 150-190° C. for 10-20 hours in the presence of solvent DMF. The catalyst consumption of the quaternary ammonium salt catalyst is 1%, the mixed trichloronitrobenzene is 450kg, and the potassium fluoride is 232kg.
[0035]After completion of the reaction, 280 kg of 3,5-dichloro-4-fluoronitrobenzene was isolated.
[0036] Then take 3,5-dichloro 4-fluoronitrobenzene as raw material in 500mL reactor, add 84g catalyst benzoyl peroxide (by raw material weight) simultaneously, feed chlorine and carry out catalytic chlorination reacti...
Embodiment 2
[0038] 500kg of 3,5-dichloro-4-fluoronitrobenzene was put into the reactor, and at the same time, in the presence of 100g of catalyst azobisisobutyronitrile, a catalytic chlorination reaction occurred with chlorine gas, and the reaction temperature was maintained at 250°C. After reacting for 4 hours, 2,4,6-trichlorofluorobenzene was obtained with a yield of 95%, and the purity of the product was 99.5% as measured by GC.
Embodiment 3
[0040] The 3,5-dichloro-4-fluoronitrobenzene of this embodiment comes from the leftovers after rectification when preparing 2,3,4-trifluoronitrobenzene, and the leftovers are 87.5% 3,5-dichloro -4-fluoronitrobenzene, the rest is 3,4-difluoro-5-chloronitrobenzene and a small amount of sulfolane, and 99.1% of 3,5-dichloro-4-fluoronitrobenzene is obtained by conventional crystallization and centrifugation benzene.
[0041] Get 3, the 3 of 3,5-dichloro-4-fluoronitrobenzene 300kg, the 3 of 5-dichloro-4-fluoronitrobenzene drops into reaction still, under the existence of 3kg catalyst crown ethers and chlorine generation catalytic chlorination reaction simultaneously, The reaction temperature was maintained at 180°C. After reacting for 5 hours, 2,4,6-trichlorofluorobenzene was obtained with a yield of 94%, and the purity of the product was 99% as measured by GC.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com