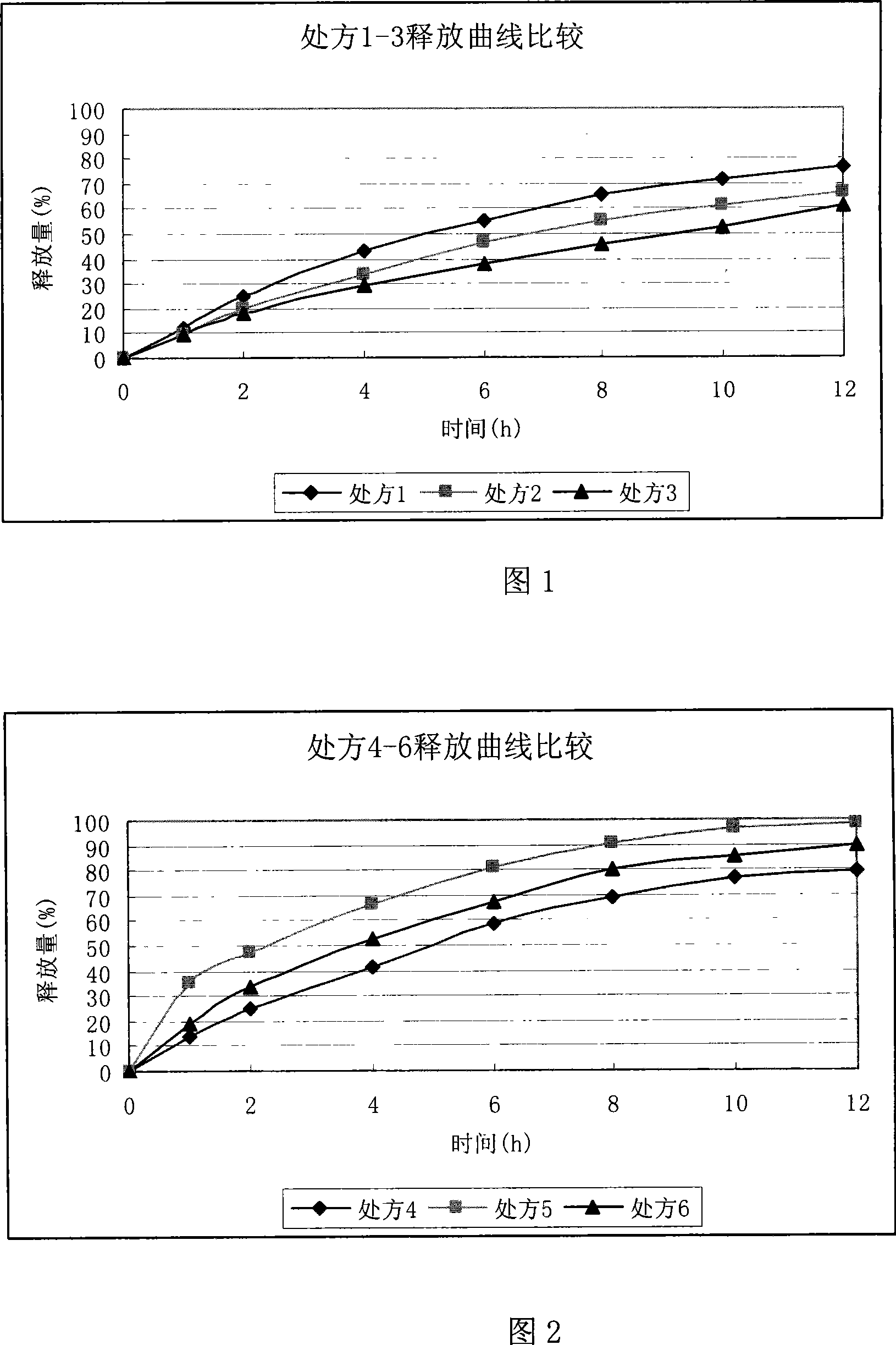
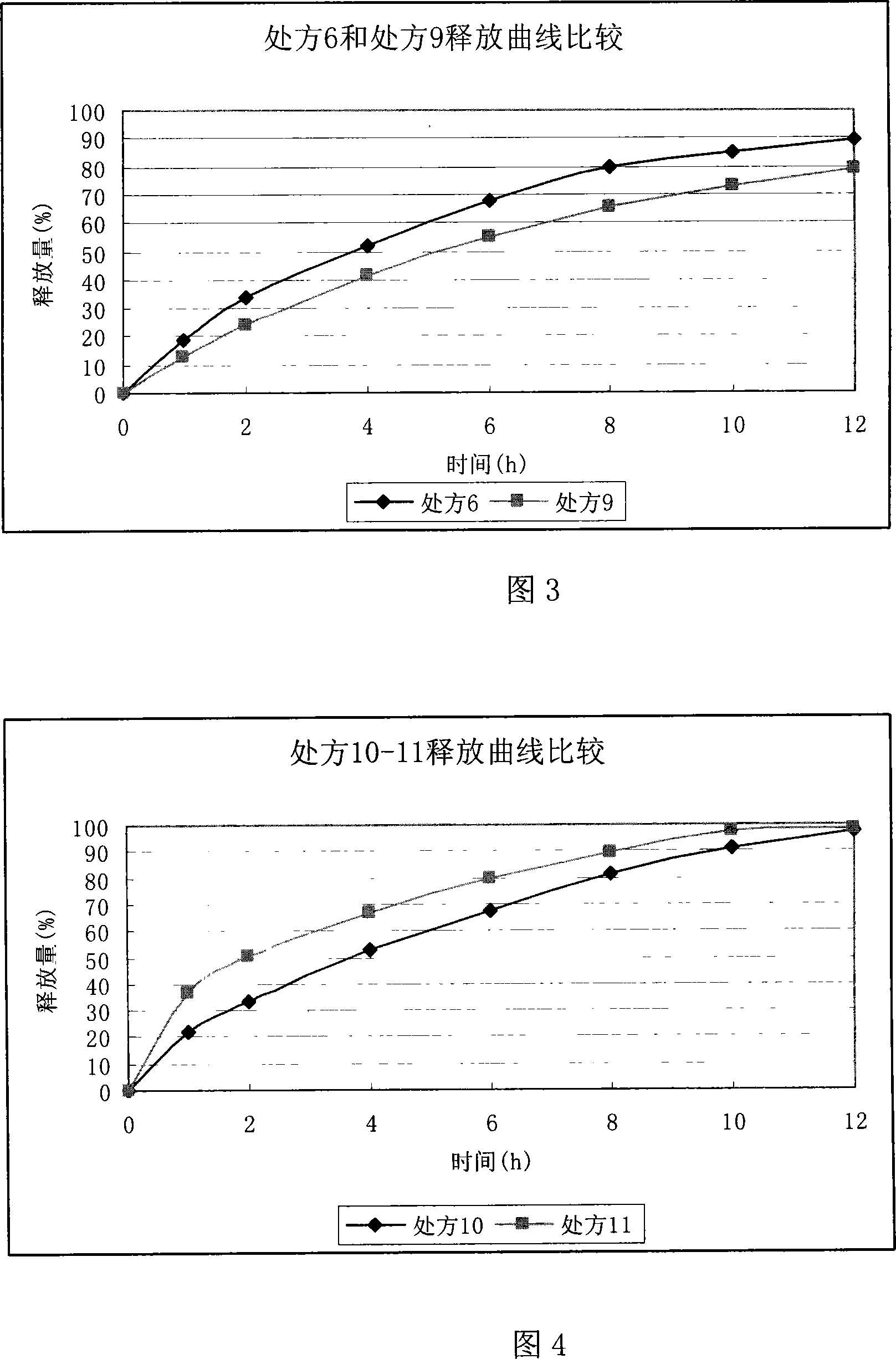
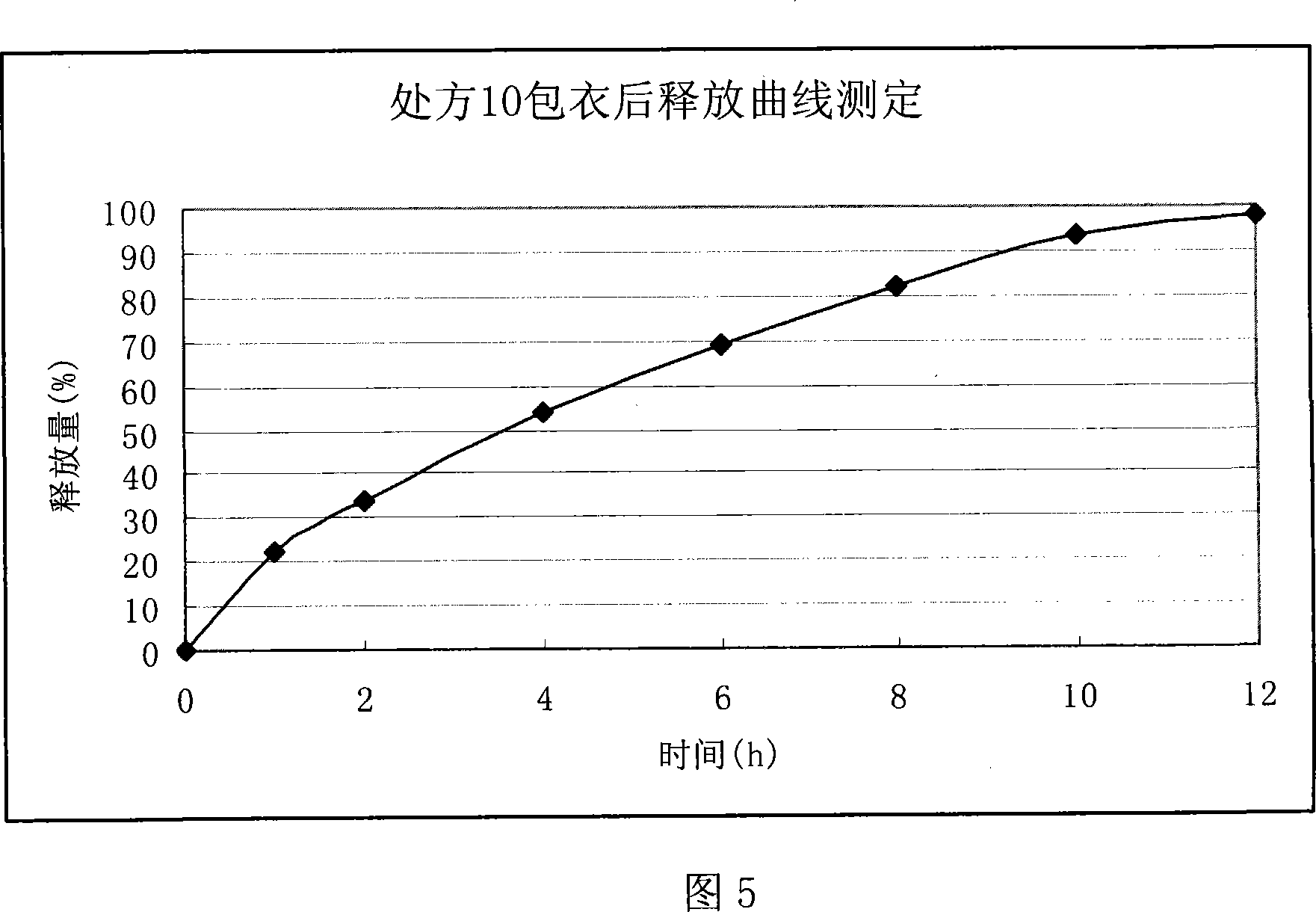
Ranolazine sustained release tablets
A ranolazine, sustained-release technology, applied in non-active ingredients medical preparations, cardiovascular system diseases, organic active ingredients, etc., can solve the problems of unstable preparations, easy moisture absorption, large fluctuations, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0151] (1) Tablet core prescription
[0152] ranolazine
500g
hypromellose K4M
hypromellose 60RT5
10% povidone K30 absolute alcohol
150g
100g
300g
Appropriate amount
20g
production
1000 pieces
[0153] (2) Prescription of coating solution
[0154] hypromellose 60RT5
Titanium dioxide
Polyethylene glycol 600
65g
20g
25g
10g
75% ethanol water
Add to 1000ml.
[0155] Preparation,
[0156] 1 smash
[0157] ranolazine, hypromellose K4M , hypromellose 60RT5 and lactose were respectively crushed through a 100-mesh sieve, and set aside.
[0158] 2. Weighing and mixing
[0159] The above-mentioned raw and auxiliary materials were weighed respectively according to the prescription amount and calculated by double checking.
[0160] 3. Granulation
[0161] Use 10% povidon...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com