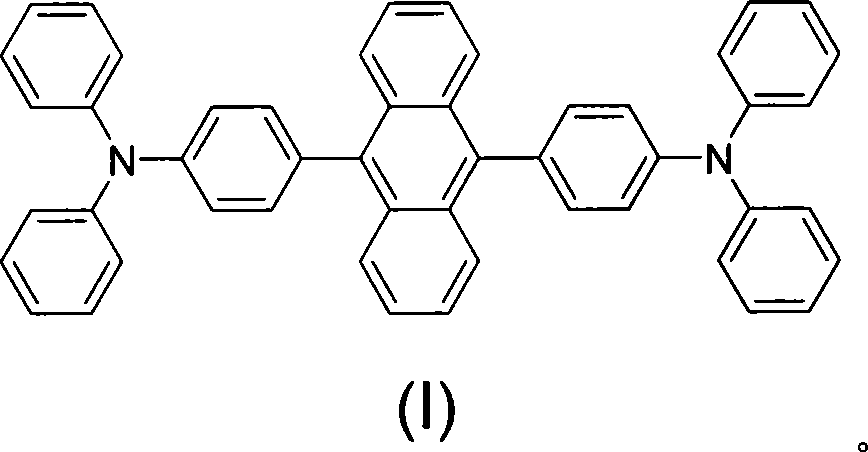
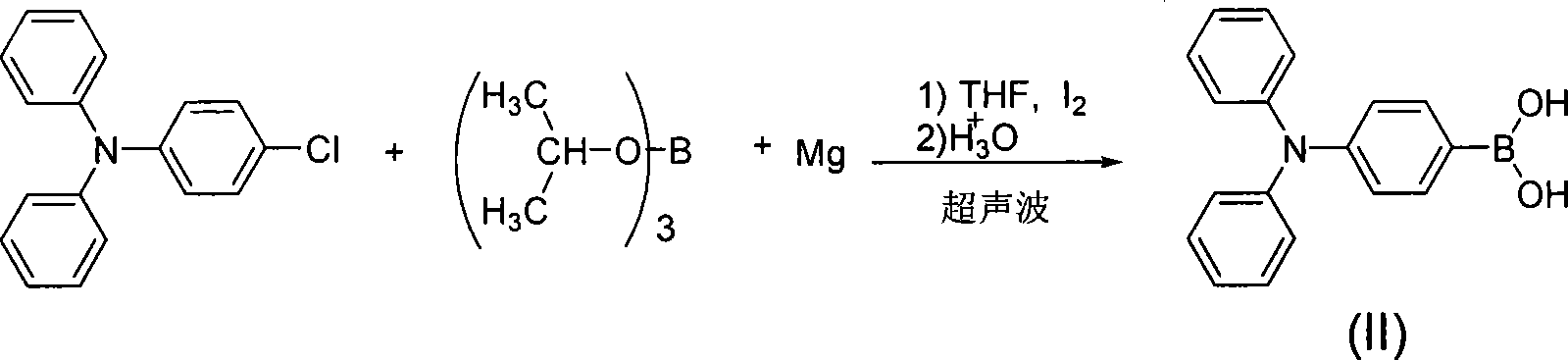Anthracene electroluminescence material containing cavity transmission group and preparation method thereof
A technology of electroluminescent material and hole transport group, applied in the direction of luminescent material, electroluminescent light source, electric light source, etc. Convenience, simple equipment, high-purity effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0015] In a 100mL single-mouth round flat-bottomed flask, add 2.55ml (11mmol) trisubstituted isopropyl borate, 2.791g (10mmol) monochlorotriphenylamine, 0.288g (12mmol) magnesium powder, 50mL of potassium hydroxide dried Tetrahydrofuran and a little iodine are used as initiators, and a reflux condenser is installed. Ultrasonic radiation is performed for 30 minutes in an ultrasonic reactor at 40°C and 175W ultrasonic power. After the reaction is completed, adjust the reaction solution to slightly acidic with 2mol / L hydrochloric acid, and stir for a while. Finally, the organic layer was separated, and a white precipitate was deposited. The aqueous layer was extracted twice with 30 mL of diethyl ether, the combined organic layers were washed with water, dried, and the solvent was removed by rotary evaporation to obtain a crude product, which was purified by recrystallization from water. The yield was 63%, 1 H-NMR (d 6 -DMSO, ppm) δ: 7.12-6.84 (m, 6H, meta carbon hydrogen of N i...
Embodiment 2
[0018] In a 100mL single-mouth round flat-bottomed flask, add 2.78ml (10mmol) trisubstituted isopropyl borate, 2.791g (10mmol) monochlorotriphenylamine, 0.36g (15mmol) magnesium powder, 50mL dried potassium hydroxide Tetrahydrofuran and a little iodine are used as initiators, and a reflux condenser is installed. Ultrasonic radiation is performed for 30 minutes in an ultrasonic reactor at 30°C and 200W ultrasonic power. After the reaction is completed, the reaction solution is slightly acidic with 2mol / L hydrochloric acid, and stirred for a while. Finally, the organic layer was separated, and a white precipitate was deposited. The aqueous layer was extracted twice with 30 mL of diethyl ether, the combined organic layers were washed with water, dried, and the solvent was removed by rotary evaporation to obtain a crude product, which was purified by recrystallization from water. The yield was 49%, 1 H-NMR (d 6 -DMSO, ppm) δ: 7.12-6.84 (m, 6H, meta carbon hydrogen of N in triphe...
Embodiment 3
[0021] According to the method of Example 1, monosubstituted triphenylamine boronic acid (II) was prepared.
[0022] In a 100mL three-necked flask, add 0.112g (0.5mmol) anhydrous palladium carbonate, 0.665g (2.5mmol) triphenylphosphine, 8.4g (25.0mmol) 9,10-di-bromoanthracene, 4.0g (50mmol) acetic acid Sodium, 15.91g (50mmol) of monosubstituted triphenylamine boronic acid, 50mL of anhydrous toluene, under nitrogen protection, heated to 90°C and stirred for 48h. After the reaction was completed, it was cooled to room temperature, and the reactant was poured into ice water, and a yellow-white precipitate was deposited. Filter, dry, dissolve with absolute ethanol, filter and dry to obtain a single yellow solid, separate the single yellow solid by column chromatography (eluent: petroleum ether: ethyl acetate = 8:1), and obtain a single yellow solid crystallization, product The rate is about 26.3%. 1 H-NMR (d 6 -DMSO, ppm) δ: 7.86-7.55 (hydrocarbon on anthracycline), δ: 7.32-6.9...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com