Insulinogen C peptide biological activity measuring method
A technology of biological activity and measurement method, which is applied in the field of chemiluminescence immunoassay to determine the biological activity of proinsulin C peptide, and can solve the problems of difficult operation control, inconsistent detection methods, and difficulty in establishing an in vitro activity measurement method.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
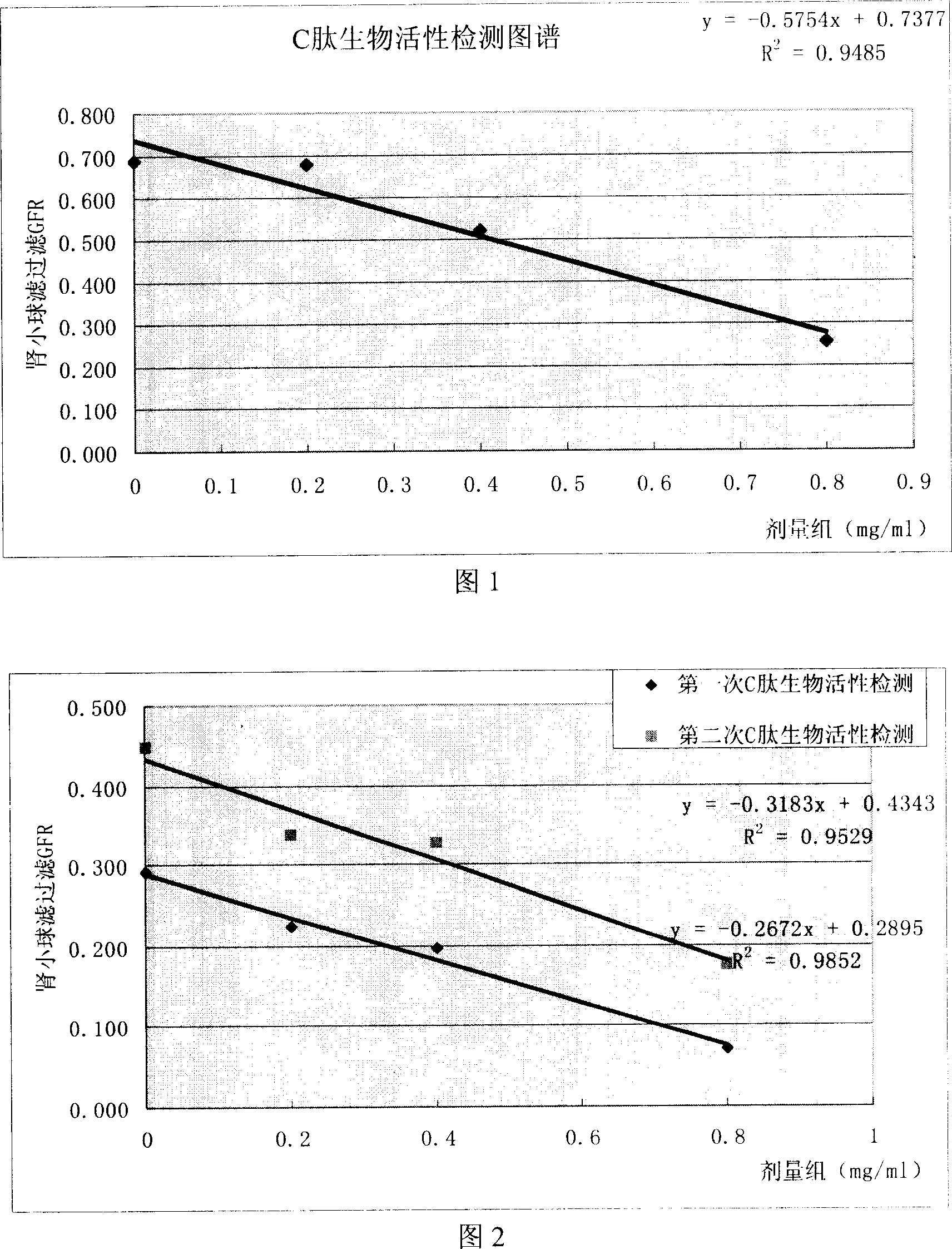
[0040] Embodiment 1. Method establishment
[0041] 1.1 Sample: self-made active reference substance (batch number 20040601, 0.8mg / bottle).
[0042] 1.2 Method introduction:
[0043] I. Grouping:
[0044] Group 1, model control group: normal saline 0.1ml / 100g (1ml / kg)
[0045] Group 2, high-dose group: C-peptide 0.8mg / kg, 0.1ml / 100g (1ml / kg), that is, 0.8mg / ml;
[0046] 3. Medium-dose group: C-peptide 0.4mg / kg, 0.1ml / 100g (1ml / kg), that is, 0.4mg / ml;
[0047] Group 4, low-dose group: C-peptide 0.2mg / kg, 0.1ml / 100g (1ml / kg), ie 0.2mg / ml.
[0048] II. Half an hour after the subcutaneous injection, the glomerular filtration rate was analyzed: for details, see Appendix 1 "Standard Operating Procedures for Bioactivity Detection of Recombinant Human Proinsulin C Peptide".
[0049] 1.2.1 Linear range:
[0050] Dose group
GFRml / min / 100gW
mouse number
Blank control group
low dose group
Middle dose group
high dose group
1
0.648
0.61 ...
Embodiment 2
[0055] Example 2. Method verification
[0056] 2.1 Sample: self-made active reference substance (batch number 20040601, 0.8mg / bottle).
[0057] 2.2 Method introduction:
[0058] I. Grouping:
[0059] Group 1, model control group: normal saline 0.1ml / 100g (1ml / kg)
[0060] Group 2, high-dose group: C-peptide 0.8mg / kg, 0.1ml / 100g (1ml / kg), that is, 0.8mg / ml;
[0061] Group 3, medium dose group: C-peptide 0.4mg / kg, 0.1ml / 100g (1ml / kg), that is, 0.4mg / ml;
[0062] Group 4, low-dose group: C-peptide 0.2mg / kg, 0.1ml / 100g (1ml / kg), ie 0.2mg / ml.
[0063] II. Half an hour after the subcutaneous injection, the glomerular filtration rate was analyzed: for details, see Appendix 1 "Standard Operating Procedures for Bioactivity Detection of Recombinant Human Proinsulin C Peptide".
[0064] 2.1 Linearity and measurement range:
[0065] Dose group
GFRml / min / 100gW
mouse number
Blank control group
low dose group
Middle dose group
high dose group
1 ...
Embodiment 3
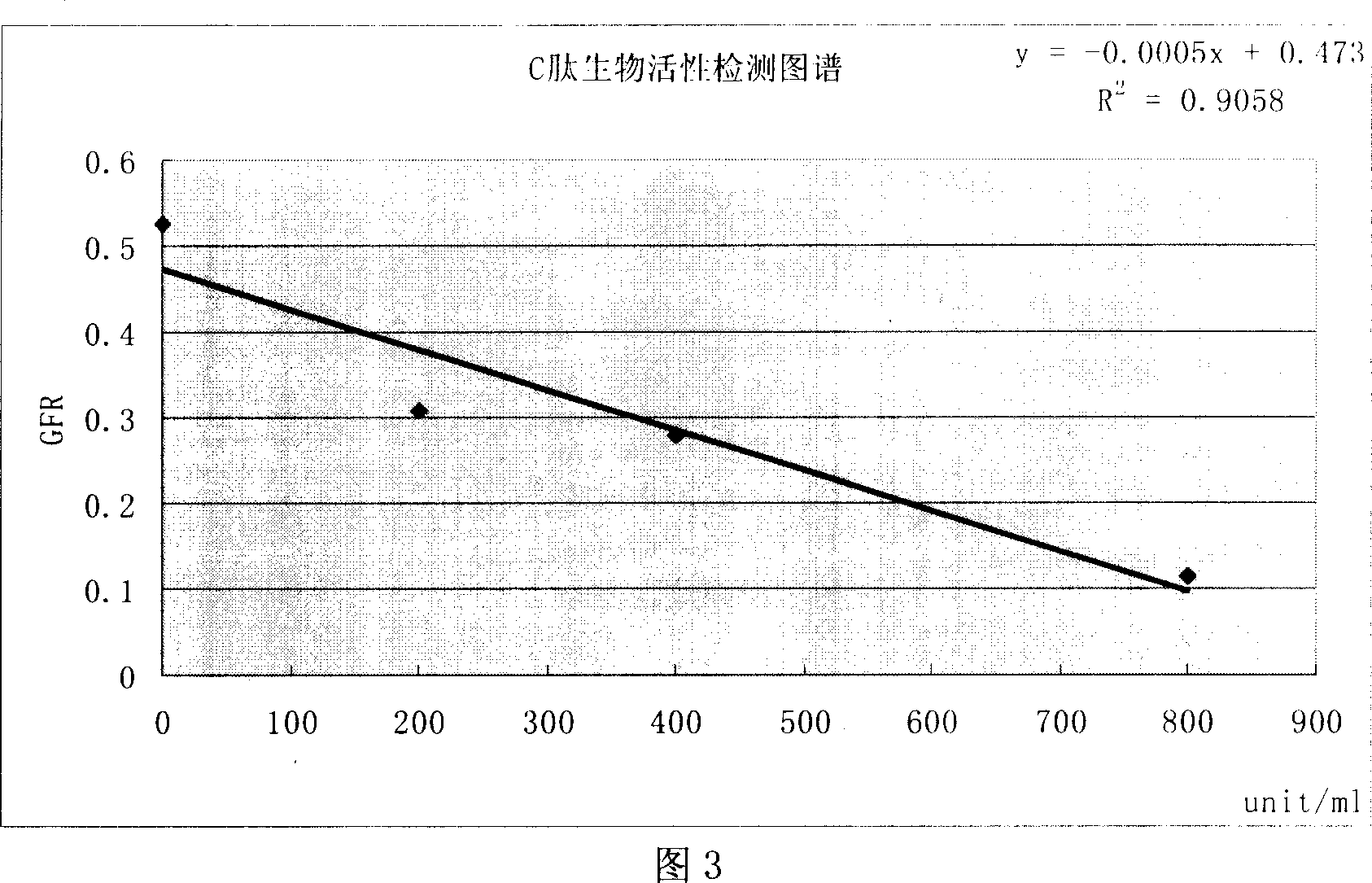
[0077] Embodiment 3 finished product detection:
[0078] 3.1 Batch number of finished product: 20050501, 20050601; specification 0.7mg / bottle (700unit / bottle)
[0079] 3.2 Standard product: self-made active reference substance (batch number 20040601, 800 unit / bottle or 0.8mg / bottle).
[0080] 3.3 Method introduction: Prepare standard curves of 0unit / ml, 200unit / ml, 400unit / ml and 800unit / ml, dilute the finished product to the linear middle of the standard curve, and conduct activity detection; see Appendix 1 "Recombinant Human Proinsulin C Peptide Biological Activity Detection Standards Operating Procedures
[0081] 3.4 Test results:
[0082] 3.4.1 Standard curve:
[0083] standard curve line
unit / ml
0
200
400
800
average glomerular filtration
RateGFR ml / min / 100gW
0.527
0.31
0.28
0.116
r=
0.952
linear equation
y=-0.0005x+0.473
[0084] See attached drawing 3.
[0085] 3.4.2 Finished prod...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com