Method for reducing or avoiding adverse effect of steroid compound
A technology of steroids and compositions, applied in the directions of steroids, drug combinations, organic chemistry, etc., can solve the problems of elevated, unknown steroid-induced intraocular pressure, etc., achieve excellent efficacy, reduce or avoid Elevated intraocular pressure, or the effect of avoiding induced intraocular pressure elevation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
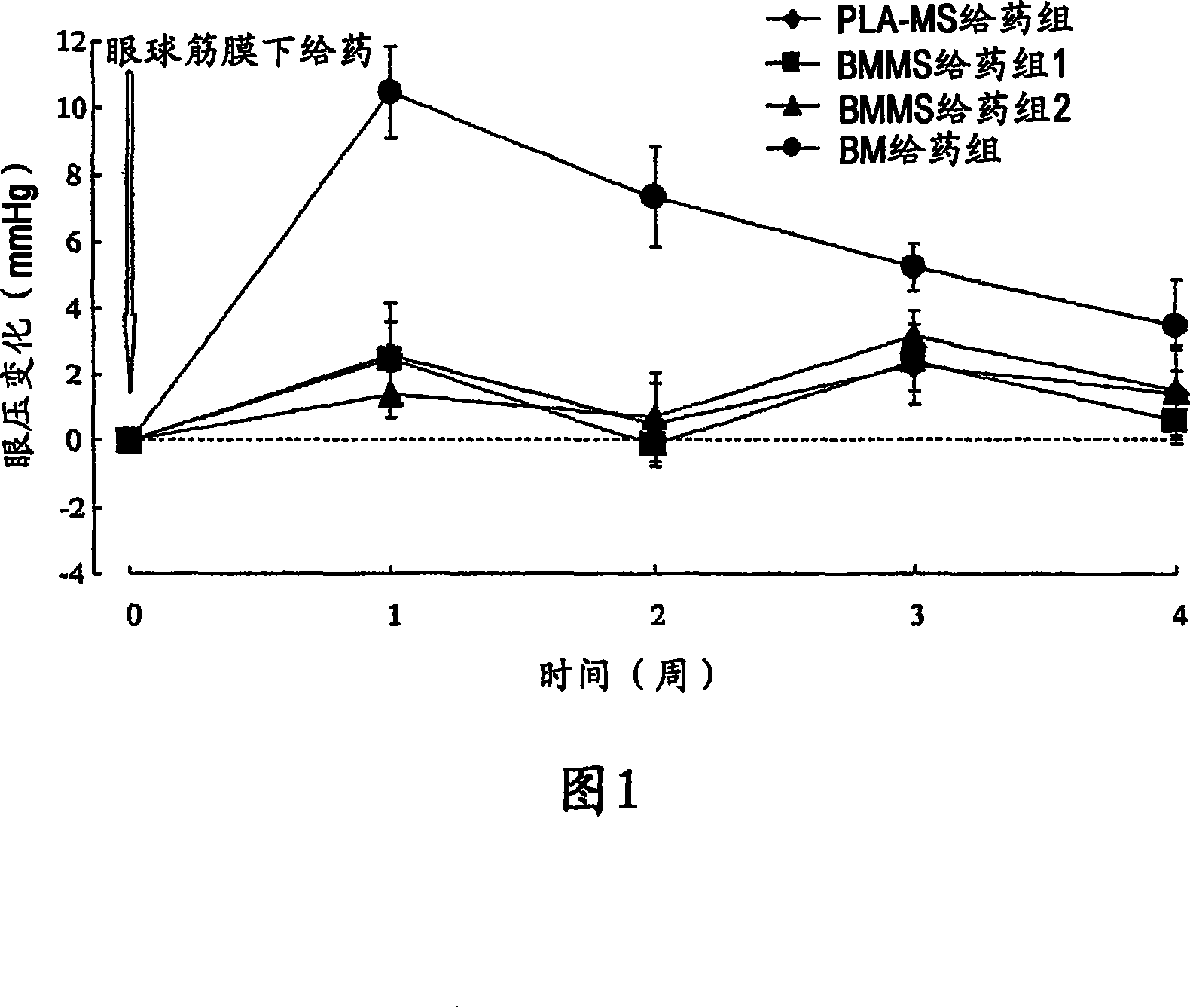
Image
Examples
manufacture example 1
[0040] Dissolve betamethasone (0.05g) and polylactic acid (0.25g) with a weight average molecular weight of about 20,000 (dispersion about 2.0) in dichloromethane (0.5mL) and benzyl alcohol (3.0mL), and the resulting solution is drug / polymer solution. A 0.2% (w / v) polyvinyl alcohol aqueous solution (400 mL) was homogenized (10000 rpm) with a homogenizer, and the drug / polymer solution was dropped thereinto. The mixture was homogenized for 10 minutes after the dropwise addition to prepare an O / W emulsion. The O / W emulsion was stirred with a stirrer for 3 hours (200 rpm). After the stirring was completed, the obtained suspension was centrifuged, and the supernatant was removed. In order to wash the precipitate, ultrapure water (30 mL) was added to disperse the precipitate, the resulting dispersion was centrifuged again, and the supernatant was removed. Repeat this operation once. The washed precipitate was sieved to obtain particles. The obtained particles were freeze-dried...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com