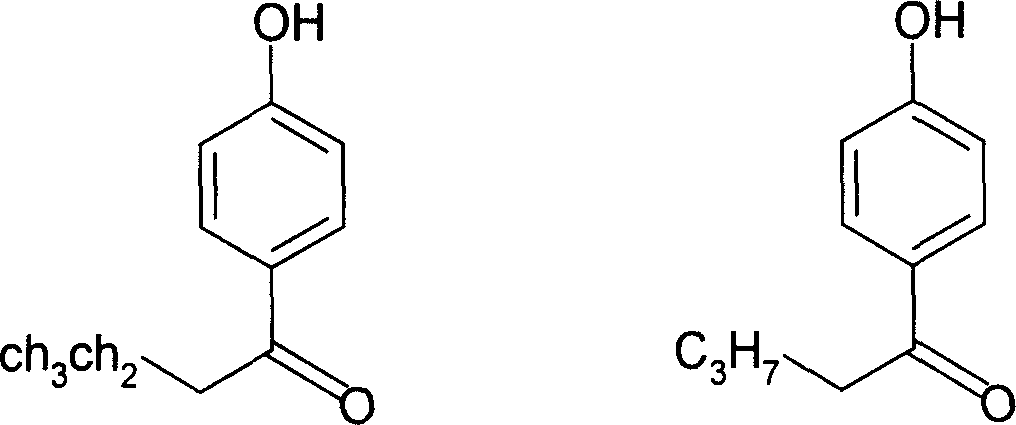
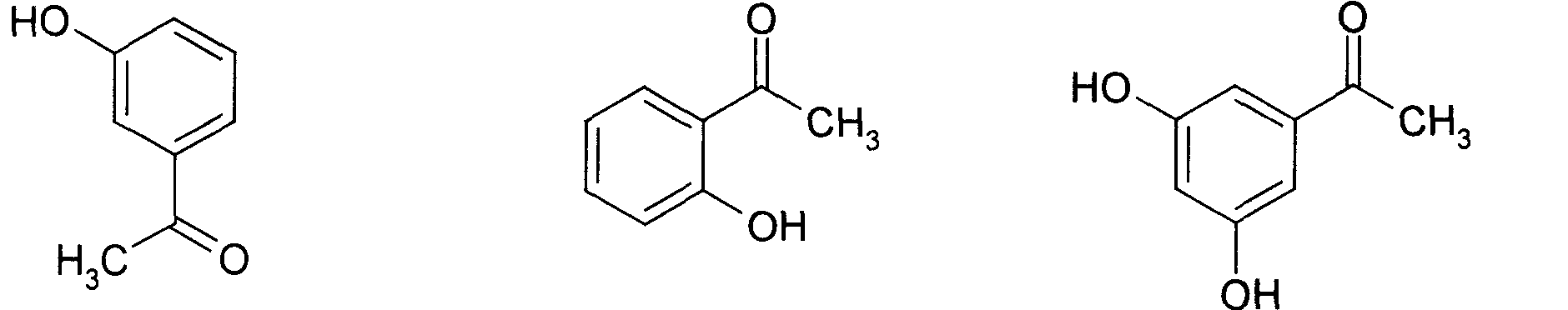
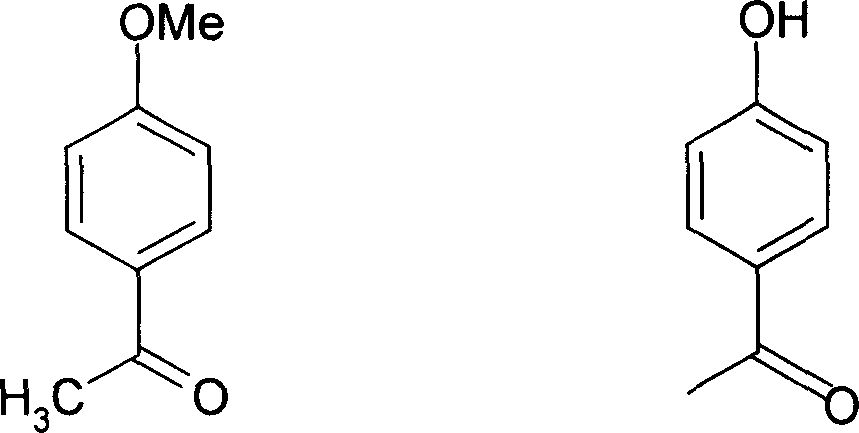Application of p-hydroxybenzene ethanone and its derivant in preparation of agentia treating gallstone disease
A technology for p-hydroxyacetophenone and m-hydroxyacetophenone, which is applied to p-hydroxyacetophenone and its derivatives in the pharmaceutical field, can solve the problems of teratogenic effect, lack of obvious curative effect, and difficulty for patients to take it for a long time. Achieve the effect of increasing bile secretion, low price and good medicinal prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Drug to be tested: p-Hydroxyvalerophenone (manufactured by Tokyo Chemical Industry Co., Ltd.)
[0046] Dosage: 19.6mg / kg;
[0047] Number of Animals: 4
[0048] a. Effects on bile secretion in rats (X±SD)
[0049]
period
Bile flow / μl
1
2
3
4
X
SD
P1
P2
Before administration
30 minutes
after administration
30 minutes
after administration
30-60 minutes
after administration
60-90 minutes
530
560
350
360
510
680
380
400
420
700
700
470
480
540
550
350
485
620
495
395
48....
Embodiment 2
[0062] Drug tested: p-nitroacetophenone (produced by Lancaster)
[0063] Dosage: 12.9mg / kg;
[0064] Number of Animals: 14
[0065] a. Effects on bile secretion in rats (X±SD)
[0066] period
Bile flow / μl
1
2
3
4
5
6
7
8
9
10
11
12
13
14
X
SD
P1
P2
Before administration
30 minutes
after administration
30 minutes
after administration
30-60 minutes
after administration
60-90 minutes
after administration
90-120 minutes
820
1300
820
700
520
1000
850
780
710
1200
900
360
100
180
340
330
600
600
500
460
420
500
500
480
...
Embodiment 3
[0084] Test drug: p-Hydroxyacetophenone (manufactured by Lancaster)
[0085] Dosage: 15mg / kg;
[0086] Number of Animals: 13
[0087] a. Effects on bile secretion in rats (X±SD)
[0088] period
Bile flow / μl
1
2
3
4
5
6
7
8
9
10
11
12
13
X
SD
P1
P2
Before administration
30 minutes
after administration
30 minutes
after administration
30-60 minutes
after administration
60-90 minutes
after administration
90-120 minutes
520
1200
850
400
500
1090
840
460
440
610
690
400
620
1220
400
600
350
600
600
580
320
300
420
460
280
280
280
680
...
PUM
| Property | Measurement | Unit |
|---|---|---|
| weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com