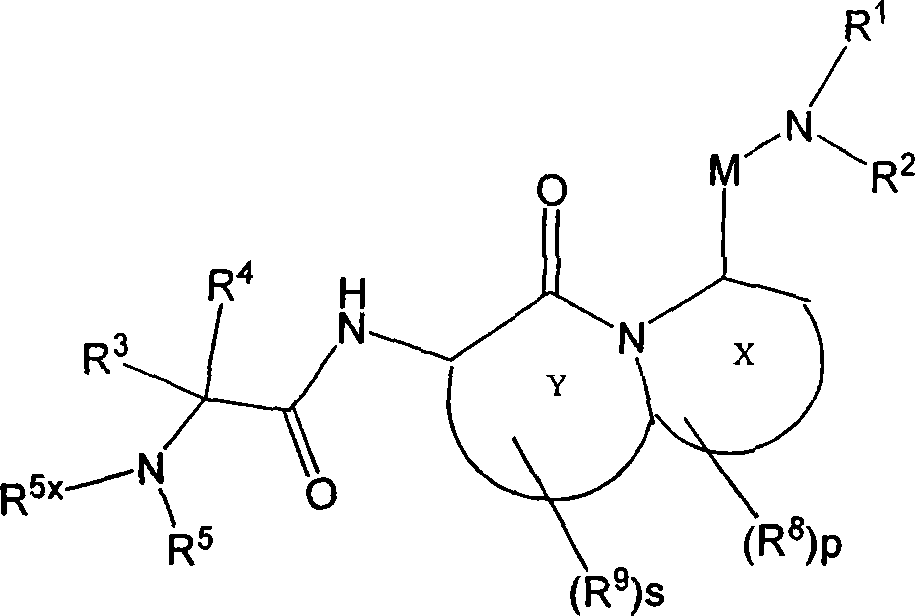
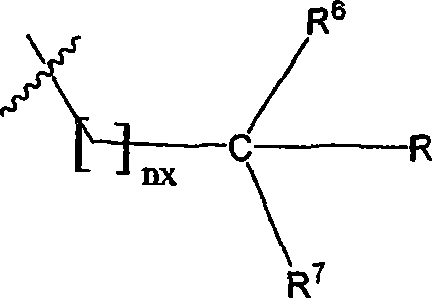
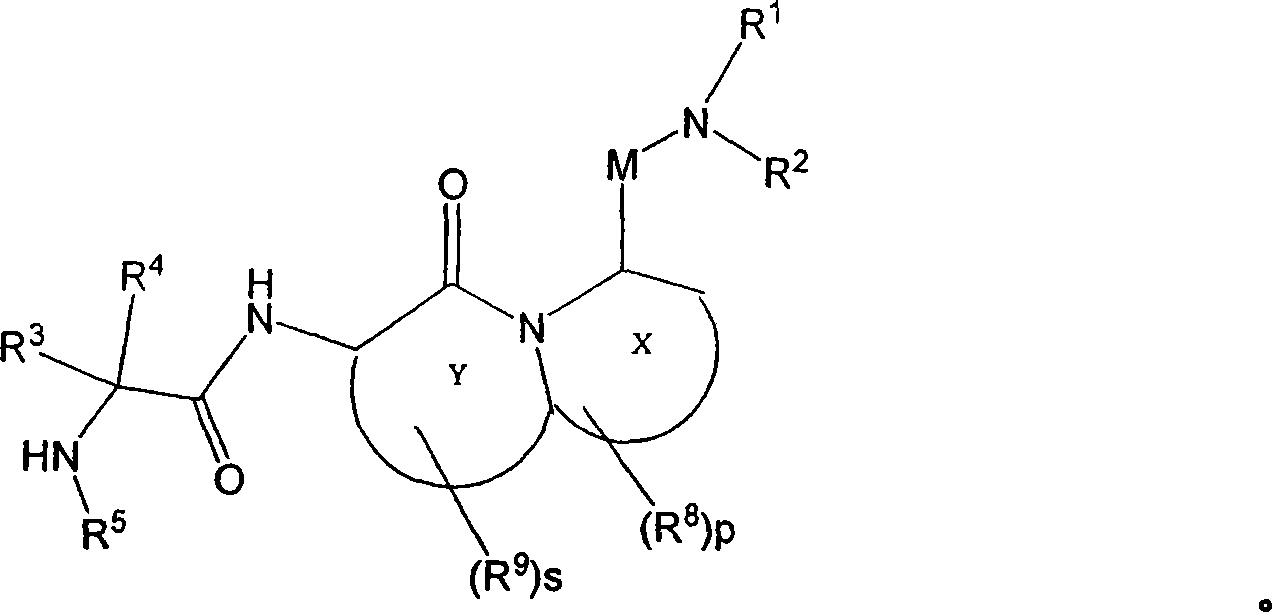
Tetrapeptide analogs
A compound and derivative technology, applied in animal repellents, peptide/protein components, plant growth regulators, etc., can solve the problem of lack of molecular specificity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0745] C. Preparation of compounds
[0746] The raw materials in the synthesis examples provided by the present invention are commercially available or obtained through literature methods. Unless otherwise indicated, all commercially available compounds were used without further purification. HPLC was done on a Zorbax C8 4.6x150mm column eluting with 10%-50% acetonitrile in water containing 0.1% TFA for 20 minutes.
[0747] The following schemes describe the general preparation of the compounds claimed in the present invention, consisting of reactions generally known to those skilled in chemical synthesis. Substituents indicated in the schemes are described elsewhere herein. It is well known to those skilled in the art that many products may exist as one or more isomers, be E / Z isomers, enantiomers and / or diastereomers.
[0748] The coupling reactions performed in the schemes below were accomplished in the presence of one of the standard peptide coupling reagents: dicyclohe...
Embodiment 1
[0902]
[0903] 2-[2-(tert-butoxycarbonyl-methyl-amino)-propionylamino]-3,3-dimethyl-butanoic acid
[0904] Part A: 2-[2-(tert-butoxycarbonyl-methyl-amino)-propionylamino]-3,3-dimethyl-butyric acid methyl ester
[0905]
[0906] To 2-(tert-butoxycarbonyl-methyl-amino)-propionic acid (1.76 g, 8.68 mmol) and 2-amino-3,3-dimethyl-butyric acid in tetrahydrofuran (THF) (100 mL) To a solution of methyl ester; hydrochloride (1.58 g, 8.70 mmol) was added 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide hydrochloride (EDCI) (3.2 g, 16.7 mmol) ) and 1-hydroxybenzotriazole monohydrate (HOBt) (1.5 g, 11.1 mmol), then N-methyl-2-pyrrolidone (2 mL) and 4-methylmorpholine (4 mL) were added. The mixture was stirred at room temperature for 18 hours. The reaction mixture was treated with water (30 mL), then partitioned with ethyl acetate / water. The organic phase was washed with 1N HCl (2×50 mL), saturated sodium bicarbonate solution (100 mL) and brine solution (50 mL), washed with Na 2 S...
Embodiment 2
[0911]
[0912] 2-[2-(tert-Butoxycarbonyl-methyl-amino)-propionylamino]-3-methyl-butanoic acid
[0913] Part A: 2-[2-(tert-Butoxycarbonyl-methyl-amino)-propionylamino]-3-methyl-butyric acid methyl ester
[0914]
[0915] To 2-(tert-butoxycarbonyl-methyl-amino)-propionic acid (5.30 g, 26.1 mmol) and 2-amino-3-methyl-butyric acid methyl ester in tetrahydrofuran (THF) (100 mL) ; Hydrochloride (6.03g, 36.0mmol) was added 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide hydrochloride (EDCI) (10.2g, 53.2mmol) and 1- Hydroxybenzotriazole monohydrate (HOBt) (5.17 g, 38.2 mmol), then N-methyl-2-pyrrolidone (6 mL) and 4-methylmorpholine (12 mL) were added. The mixture was stirred at room temperature for 18 hours. The reaction mixture was treated with water (30 mL), then partitioned with ethyl acetate / water. The organic phase was washed with 1N HCl (2×75 mL), saturated sodium bicarbonate solution (150 mL) and brine solution (50 mL), washed with Na 2 SO 4 Drying and evaporation t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com