2'-fluoroncucleosides
A technology for replacing nucleosides and bases, which is applied in the field of 2'-fluoro nucleosides and its preparation, and can solve problems such as cleavage of glucosyl bonds and difficulties in fluorine groups.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
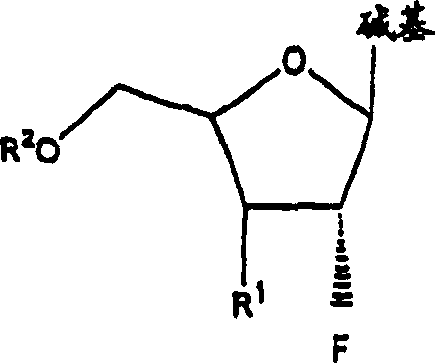
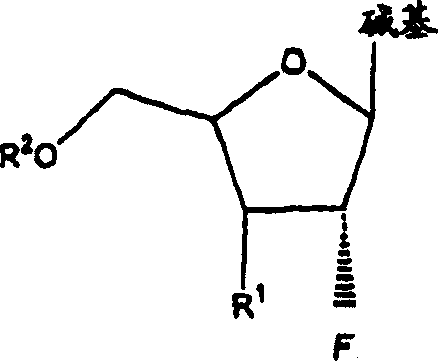
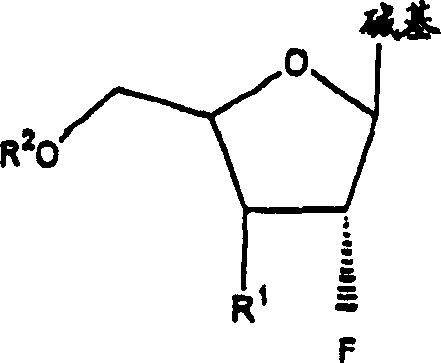
[0073] The invention disclosed herein relates to a compound, method and composition for the treatment of HIV, hepatitis (B or C) or abnormal cell proliferation in a human or other animal host comprising the use of An effective amount of 2'-fluoro-nucleoside, a pharmaceutically acceptable derivative (including compounds that have been alkylated or acylated at the 5'-position or at a purine or pyrimidine), or a pharmaceutically acceptable salt thereof. Compounds of the invention possess antiviral (ie anti-HIV-1, anti-HIV-2 or anti-hepatitis (B or C)) activity, or antiproliferative activity, or are metabolized to compounds having these activities.
[0074] In summary, the present invention includes the following features:
[0075] (a) β-L and β-D-2'-fluoronucleosides, as described herein, and pharmaceutically acceptable derivatives and pharmaceutically acceptable salts thereof,
[0076] (b) β-L and β-D-2'-fluoronucleosides, as described herein, and pharmaceutically acceptable de...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com