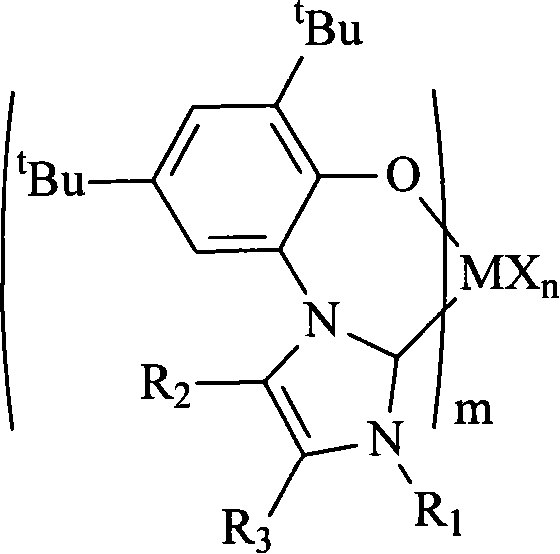
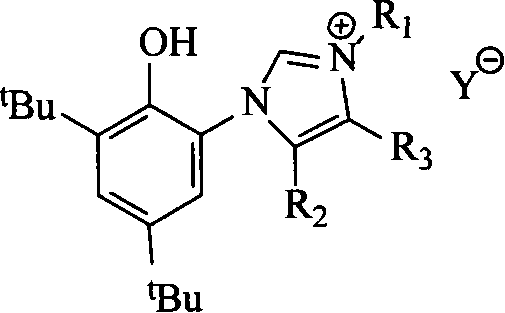
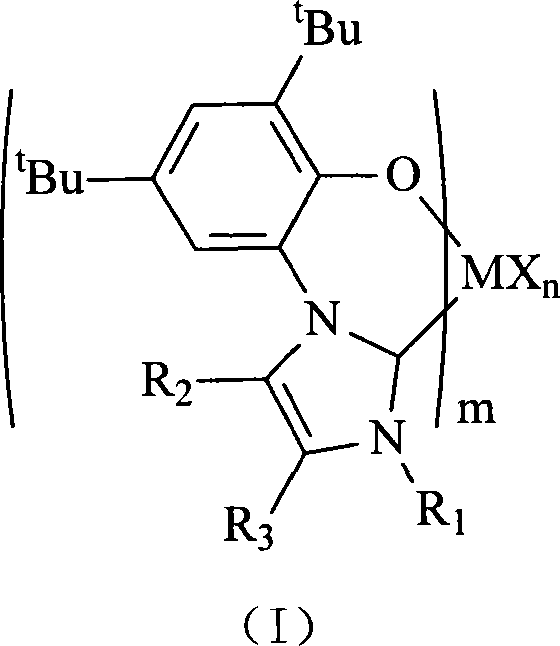
Transition metal polymerization catalyst containing CO bidentate ligand
A transition metal and catalyst technology, applied in the field of ortho-hydroxyaryl-substituted N-heterocyclic carbene metal compounds, can solve the problems of no reported results and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0067] Example 1 Synthesis of Complex C1
[0068]
[0069] In a 100 mL reaction flask, 1.2 mL (1.7 N, 2.0 mmol) of n-BuLi in hexane was added dropwise to a suspension of 0.41 g (1.0 mmol) of ligand L1 in THF (30 mL) at 0°C , stirred at 0°C for 0.5 hours, slowly warmed to room temperature, and then added 0.33 g (0.5 mmol) of NiCl 2 (PPh 3 ) 2 , stirred overnight at room temperature. The solvent was drained, separated by alumina column chromatography, and 1:1 CH 2 Cl 2 / petroleum ether, and the obtained components were drained of solvent to obtain 0.33 g of complex C1, with a yield of 92%.
[0070] Elemental analysis: measured (calculated) 70.35 (70.68); H, 8.57 (8.76); N, 7.46 (7.85);
[0071] 1 H NMR (400MHz, CDCl 3 ): δ7.19(d, 1H, im-H, J=1.69Hz), 7.12(s, 1H, Ar-H), 7.05(s, 1H, Ar-H), 7.02(d, im-CH, J=2.04Hz), 1.66(s, 9H, C(CH) 3 ), 1.54(s, 9H, C(CH) 3 ), 1.37(s, 9H, C(CH) 3 );
Embodiment 2
[0072] Example 2 Synthesis of Complex C2
[0073]
[0074] In a 100 mL reaction flask, 1.2 mL (1.7 N, 2.0 mmol) of n-BuLi in hexane was added dropwise to a suspension of 0.44 g (1.0 mmol) of ligand L2 in THF (30 mL) at 0°C , stirred at 0°C for 0.5 hours, slowly warmed to room temperature, and then added 0.33 g (0.5 mmol) of NiCl 2 (PPh 3 ) 2 , stirred overnight at room temperature. The solvent was drained, separated by alumina column chromatography, and 1:1 CH 2 Cl 2 / petroleum ether rinsing, the obtained components were drained of solvent to obtain 0.37g of complex C2, and the yield was 95%.
[0075] Elemental analysis: measured (calculated) 72.07 (72.15); H, 8.95 (8.69); N, 7.52 (7.32);
[0076] 1 H NMR (300MHz, CDCl 3 ): δ7.22(d, 1H, im-CH, J=1.94Hz), 7.19(d, 1H, Ar-H, J=2.40Hz), 7.04(d, 1H, Ar-H, J=2.40Hz ), 6.93(d, 1H, im-CH, J=1.96Hz), 3.52(m, 1H, N(CH)(CH2)5), 1.55(s, 9H, C(CH) 3 ), 1.34(s, 9H, C(CH) 3 ), 1.75~1.26 (m, 10H, (CH 2 ) 5 );
Embodiment 3
[0077] Example 3 Synthesis of Complex C3
[0078]
[0079] In a 100 mL reaction flask, 1.2 mL (1.7 N, 2.0 mmol) of n-BuLi in hexane was added dropwise to a suspension of 0.47 g (1.0 mmol) of ligand L3 in THF (30 mL) at 0°C , stirred at 0°C for 0.5 hours, slowly warmed to room temperature, and then added 0.33 g (0.5 mmol) of NiCl 2 (PPh 3 ) 2 , stirred overnight at room temperature. The solvent was drained, separated by alumina column chromatography, and 1:1 CH 2 Cl 2 / petroleum ether rinsing, and the obtained components were drained of solvent to obtain 0.41 g of complex C3, with a yield of 97%.
[0080] Elemental analysis: measured (calculated) 74.08 (74.55); H, 8.21 (7.94); N, 6.53 (6.69);
[0081] 1 H NMR (300MHz, CDCl 3 ): δ 7.26(s, 1H, Ar-H), 6.91(s, 1H, im-CH), 6.90(s, 1H, im-CH), 6.72(s, 1H, Ar-H), 6.64(s , 2H, Ar-H), 3.53(s, 3H, Ar-CH 3 ), 2.26(s, 3H, Ar-CH 3 ), 1.80 (s, 3H, Ar-CH 3 ), 1.56(s, 9H, C(CH) 3 ), 1.45(s, 9H, C(CH) 3 );
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com