Application of cycloartane type triterpenoids compound of cimicifuga rhizome for anti-osteoporosis and menopausal syndrome
A technology for cyclic jackfruit alkane and jackfruit alkane, applied in the field of triterpenoids, can solve the problems of no indication of active ingredients, no report of cichoside compounds, risk of breast cancer, endometrial cancer, etc., and achieve the effect of less side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
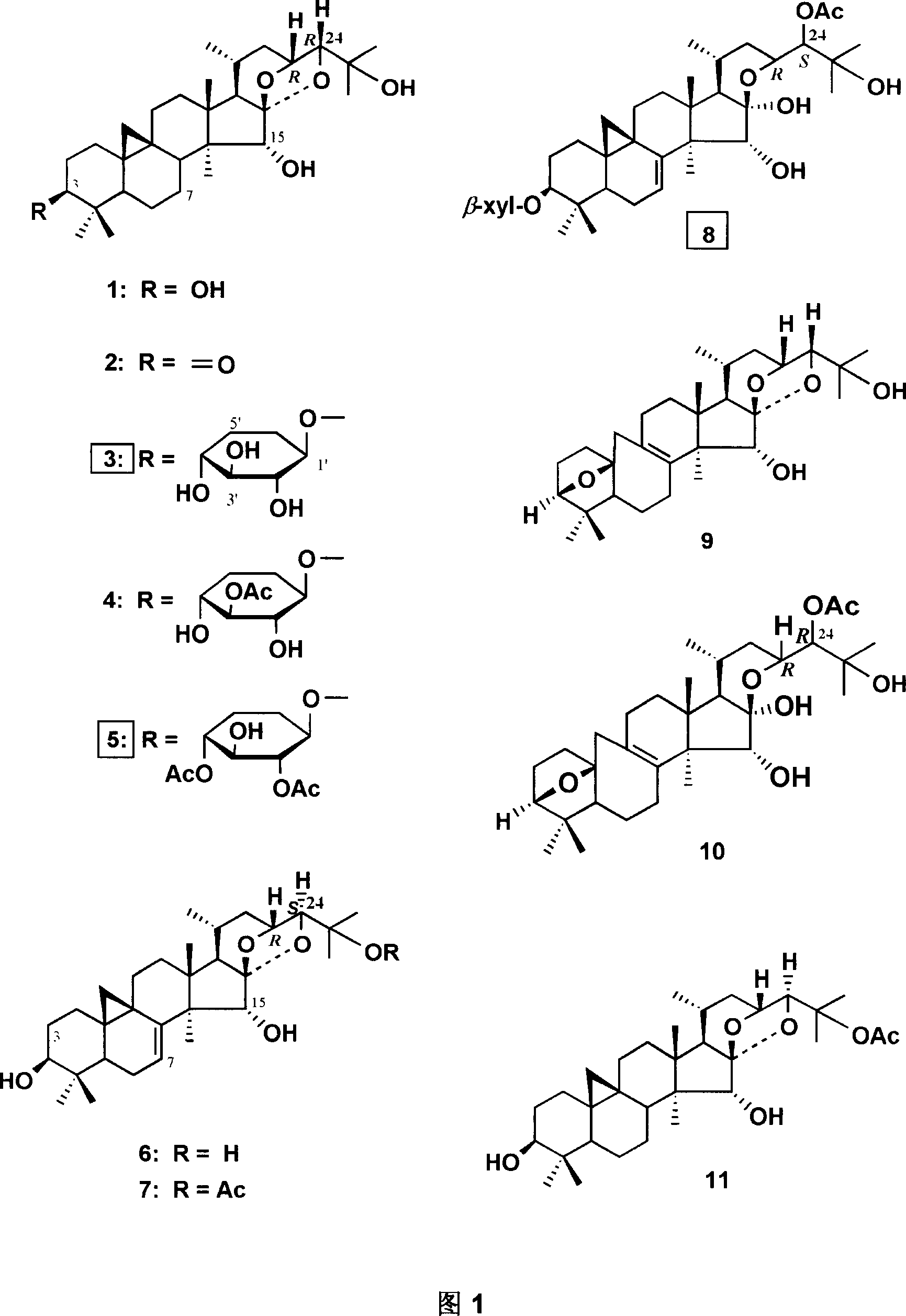
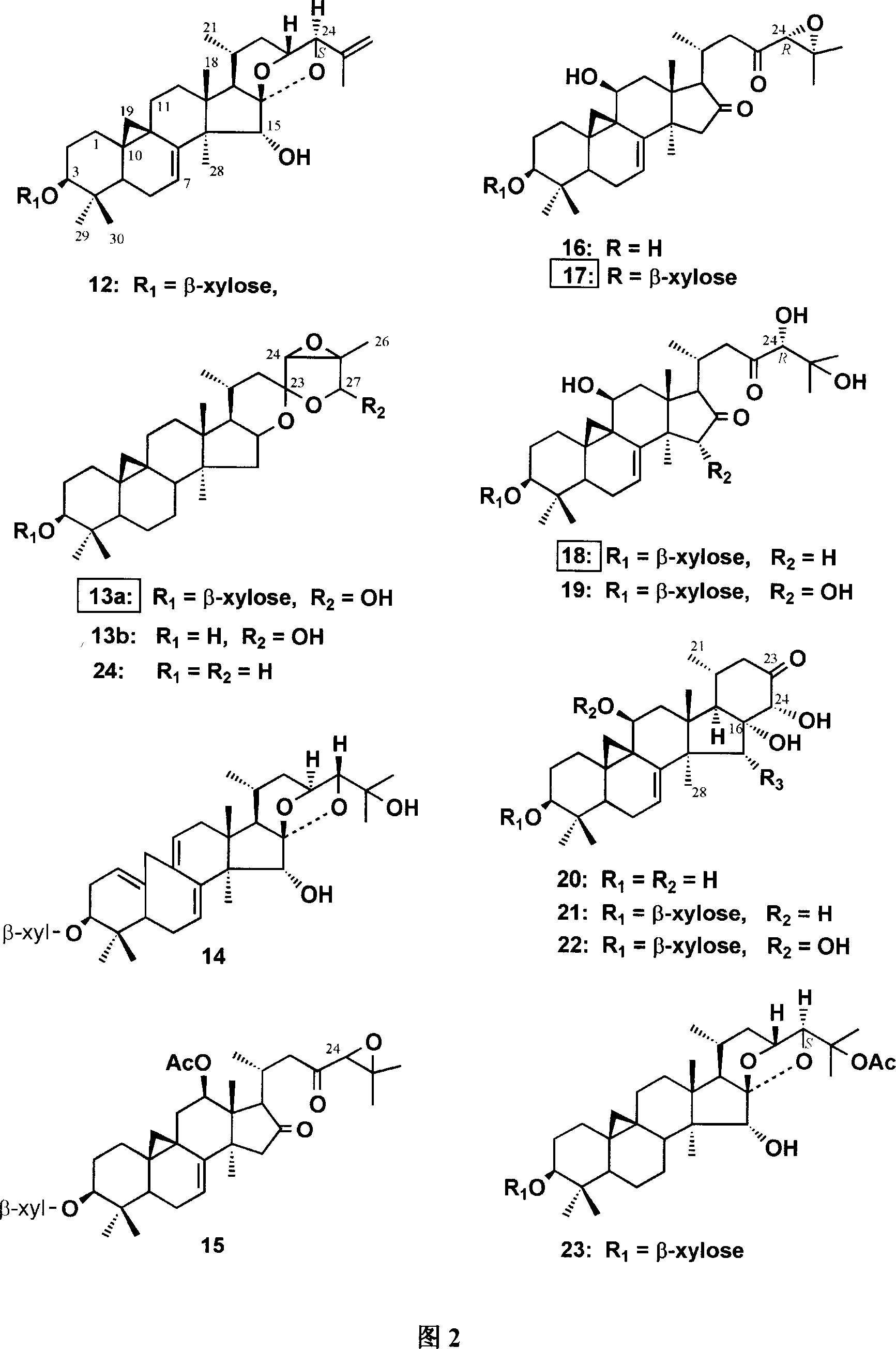
[0034] Example 1: Compound extraction and separation and structural identification
[0035] The extraction method of the extract of Cimicifuga cyclopine-type triterpenoids produced in China: the dried rhizomes and Fibrous roots, crushed, heated to reflux with 50-70% ethanol or propanol, extracted 1-4 times. Combine the extracts and concentrate under reduced pressure to remove the solvent in the extracts to obtain ethanol or propanol extract. Dissolve the extract in distilled water or a small amount of 50% ethanol, suspend it in distilled water, add petroleum ether solvent for 1-4 times of extraction, and similarly distill the suspension of the extract in distilled water extracted by petroleum ether with acetic acid Ethyl esters and other esters are extracted for 1-4 times to obtain ester extracts. Concentrate under reduced pressure to remove the solvent in these extracts to obtain ester extracts. Ester extract is dissolved in 95-100% ethanol, by gac column chromatography, r...
Embodiment 2
[0067] Example 2: Anti-resorptive activity
[0068] The compounds in Figures 1 and 2 were prepared in the three concentrations described in the table below with Ham's F-12 culture medium. Use suckling mice 2 days after birth, and inject subcutaneously 45 CaCl 2 (2 Ci), after 2 days, remove the skull, use Ham's F-12 culture medium, at a temperature of 37 ° C, CO 2After pre-cultivation for 24 hours under the condition of 5%, replace the new culture medium, and add 1,25 dihydroxy active vitamin D3 (1,25-(OH) 2 VD 3 , final concentration 10 -10 M) and each compound (see the table below for the final concentration, 200; 20 and 2 micromolar) continued to culture for 3 days under the same conditions as the pre-cultivation, and measured the amount of 45 Ca (measured value 1). Renew this culture medium, continue to cultivate under the same conditions as pre-cultivation again for 3 days, and measure the amount released into the culture medium in the same way. 45 Ca (measured valu...
Embodiment 3
[0074] Example 3: Anti-resorptive activity
[0075] The compound in Example 2 was formulated into 3 kinds of concentrations described in the table below like Example 2, 200; 20 and 2 micromoles.
[0076] 45 Ca release rate (%)
1,25 dihydroxyactive vitamin
D3
58.15±1.78
Normal group (without 1,25 dihydroxyl activity
vitamin D3 and compounds)
36.15±1.57 **
compound
2 micromoles (g
Compound concentration is 2 / 3
micromole)
20 micromoles (varies
Compound concentration is 20 / 3
micromole)
200 micromoles (each
Compound concentration is 200 / 3
micromole)
3, 5, 8 mixture
39.18±1.04 **
37.61±3.66 **
14.84±0.10 **
13a, 17, 18 mixture
40.22±2.61 *
29.78±2.30 **
14.10±0.10 **
[0077] Data are expressed as Mean ± SE. Compared with the 1,25 dihydroxyactive vitamin D3 group, there was a significant difference, * , p...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com