Interfusion protein between diphtheria toxin and GM CSF mutant, coded gene and application
A technology of GM-CSF and diphtheria toxin, which is applied in the fusion protein of diphtheria toxin and GM-CSF and its coding gene and application field, can solve the problems of high resistance to development, low expression level, unfavorable mass industrial production, etc., and achieve survival The effect of time improvement and high expression volume
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] Embodiment 1, diphtheria toxin and GM-CSF fusion protein DT 386 -Expression of GMCSF
[0055] 1. The fusion protein DT of diphtheria toxin and GM-CSF 386 -Expression of GMCSF
[0056] 1. DT 386 - Construction of GMCSF expression plasmid
[0057] The ORF of the human GM-CSF gene composed of E. coli preferred codons (the gene is called GM-CSFm) was artificially synthesized and inserted into the EcoR V site of pcDNAII (Invitrogen, V40020) to obtain the recombinant vector pcDNAII-GMCSFm. Wherein, the nucleotide sequence of the ORF of GM-CSFm is sequence 3 in the sequence listing (sequence 3 consists of 390 nucleotides).
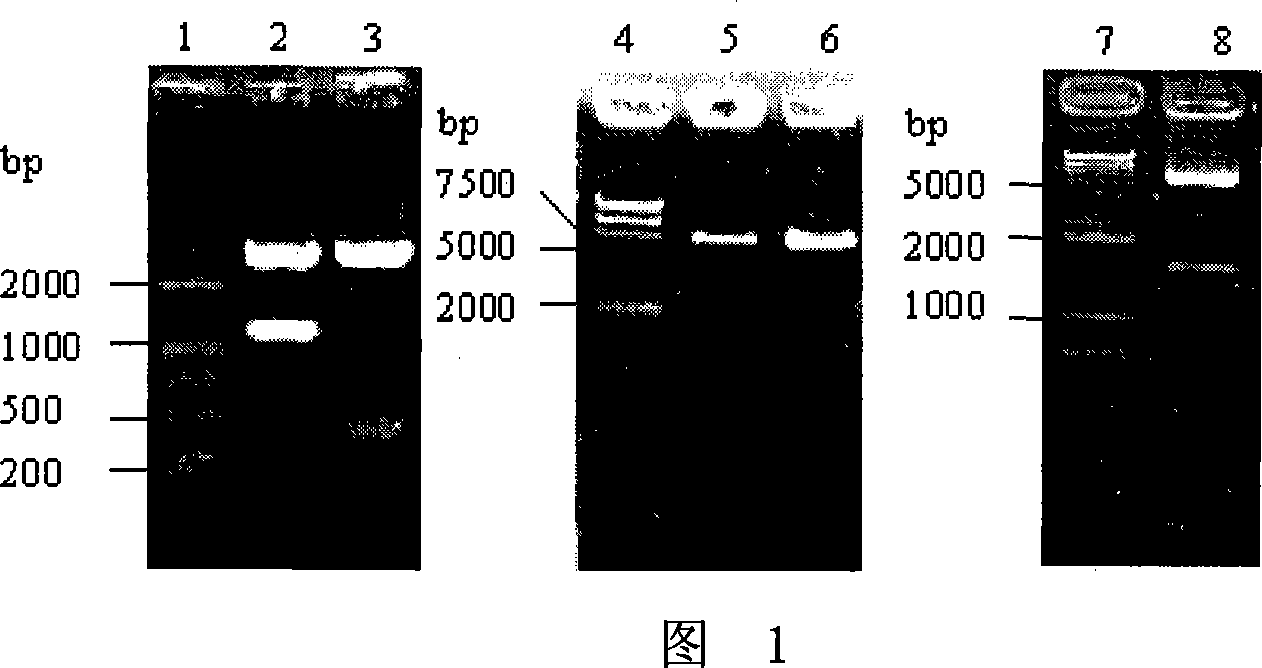
[0058] Using pcDNAII-GMCSFm as a template, primer GFS with SphI restriction site and primer GFP14 with BamH I restriction site were used for PCR amplification. The length of the PCR product is about 400bp, and the blunt end connection is inserted into the EcoR V site of the pcDNAII plasmid. The results of Sph I and Bam HI double enzyme digestion and ...
Embodiment 2
[0085] Embodiment 2, GMCSF-DT 386 activity assay
[0086] 1. GMCSF-DT 386 Specific cytotoxic effect on HL60
[0087] The HL60 cell line belongs to promyelocytic leukemia cells and is a commonly used cell line for leukemia research. Because there are a large number of GM-CSF receptors on the cell surface, it can be used as the target cell of the immunotoxin in this study to detect the cytotoxic activity of the toxin. Determination of immunotoxin GMCSF-DT by MTS method 386 Cytotoxicity of purified fractions of P1 and P2 against HL60.
[0088] The MTS method was operated according to the instructions of the MTS detection kit of Promega Company. Collect HL60 cells in the logarithmic growth phase and adjust to 5×10 with RPMI 1640 culture medium with 10% FCS 5 cells / ml, insert 0.1ml / well into 96-well cell culture plate, culture for 24h, add 0.1ml of GMCSF-DT 386 Purified fraction of P1 or 0.1 ml of GMCSF-DT 386 P2 purified fraction or 0.1ml of DT 386 -GMCSF or 0.1ml of DT ...
Embodiment 3
[0104] Embodiment 3, GMCSF-DT 386 Mechanism of Cytotoxicity
[0105] 1. Morphological study of apoptotic cells
[0106] HL60 cells were treated with 10 5 A / ml concentration into 25cm 2 Culture flasks or 6-well plates for 24 hours. Join GMCSF-DT 386 Purified fraction of P1 or GMCSF-DT 386 The P2 purified fraction to a final concentration of 0; 1×10 -9 mol / L; 1×10 -8 mol / L; 1×10 -7 After mol / L treatment for 48 hours, cells were collected by centrifugation at 1,000 rpm for 10 minutes, washed once with PBS, centrifuged at 1,000 rpm for 10 minutes, resuspended in serum-free medium, and stained with 10 μg / ml Hoechst 33342. Unstained or stained cell drops were observed and photographed under a fluorescent microscope.
[0107] Diphtheria toxin kills cells by inactivating ADP-ribosylated EF-2 (elongation factor), thereby inhibiting cell protein synthesis and eventually causing cell death. This experiment first observed GMCSF-DT morphologically 386 Specific cytotoxicity again...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com