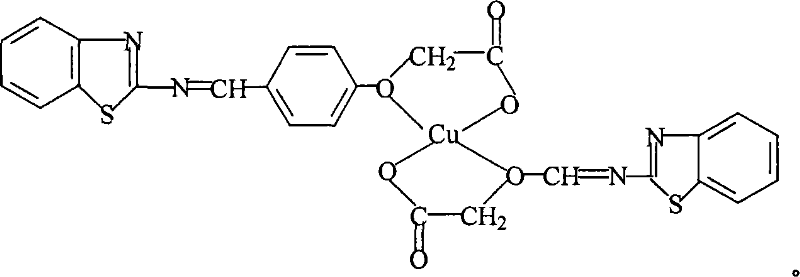
4-(2-Benzothiazolyl aminoiminomethyl) phenoxyacetic acid and cupric complexes and preparation methods thereof
A technology of benzothiazolyliminomethyl and phenoxyacetic acid, which is applied in the field of 4-phenoxyacetic acid and its copper complexes and their preparation, can solve the problems such as the antibacterial performance needs to be improved, and achieves the improvement of the antibacterial and antifungal performance. , the effect of increasing the coordination group and enriching the coordination mode
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] (1) Preparation of 4-(2-benzothiazolyl iminomethyl)phenoxyacetic acid Take p-formylphenoxyacetic acid 7.2g (0.04mol), 2-aminobenzothiazole 6.0g (0.04mol) and methyl Add 174.3mL (2.0mol) of acetone into a 500mL round-bottomed flask, stir, and reflux at a constant temperature of 80°C for 2h; stop the reaction, cool to room temperature and filter, and the obtained solid is 4-(2-benzothiazolyl Aminomethyl) phenoxyacetic acid crude product; the filtrate is transferred to the distillation unit, distilled and reclaimed methyl acetone 160mL, the residual solid matter is 4-(2-benzothiazolyl iminomethyl) phenoxyacetic acid crude product The 4-(2-benzothiazolyl iminomethyl) phenoxyacetic acid crude product of twice gained is merged, and is dried to constant weight first in the vacuum drying device of 40 ℃, then recrystallizes with 200mL ethanol, again in Dry to constant weight in a vacuum drying device at 40° C. to obtain 10.5 g of 4-(2-benzothiazolyl iminomethyl) phenoxyacetic ac...
Embodiment 2
[0047] (1) Preparation of 4-(2-benzothiazolyl iminomethyl)phenoxyacetic acid takes p-formylphenoxyacetic acid 7.2g (0.04mol), 2-aminobenzothiazole 6.0g (0.04mol) and acetone 294.9 Add mL (4.0mol) into a 500mL round-bottomed flask, stir, and reflux at a constant temperature of 70°C for 5 hours; stop the reaction, cool to room temperature and filter, and the obtained solid is 4-(2-benzothiazolyl iminomethyl base) phenoxyacetic acid crude product; the filtrate is transferred to a distillation apparatus, distilled and reclaimed acetone 280mL, and the residual solid matter is 4-(2-benzothiazolyl iminomethyl) phenoxyacetic acid crude product; The crude products of 4-(2-benzothiazolyl iminomethyl)phenoxyacetic acid obtained were combined, first dried to constant weight in a vacuum drying device at 45°C, then recrystallized with 200mL ethanol, and then recrystallized in a vacuum at 45°C. Dry to constant weight in a drying device to obtain 11.3 g of 4-(2-benzothiazolyliminomethyl)pheno...
Embodiment 3
[0050] (1) Preparation of 4-(2-benzothiazolyl iminomethyl)phenoxyacetic acid Take p-formylphenoxyacetic acid 7.2g (0.04mol), 2-aminobenzothiazole 6.0g (0.04mol) and methanol 242.4 Add mL (6.0mol) into a 500mL round bottom flask and stir, and reflux at a constant temperature of 60°C for 8h; stop the reaction, cool to room temperature and filter, the obtained solid is 4-(2-benzothiazolyl iminomethyl base) crude product of phenoxyacetic acid; transfer the filtrate to a distillation apparatus, distill and reclaim 230 mL of methanol, and the residual solid matter is the crude product of 4-(2-benzothiazolyl iminomethyl) phenoxyacetic acid; The crude products of 4-(2-benzothiazolyl iminomethyl)phenoxyacetic acid obtained were combined, first dried to constant weight in a vacuum drying device at 40°C, then recrystallized with 200mL ethanol, and then recrystallized in a vacuum at 40°C. Dry to constant weight in a drying device to obtain 11.6 g of 4-(2-benzothiazolyliminomethyl)phenoxya...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com