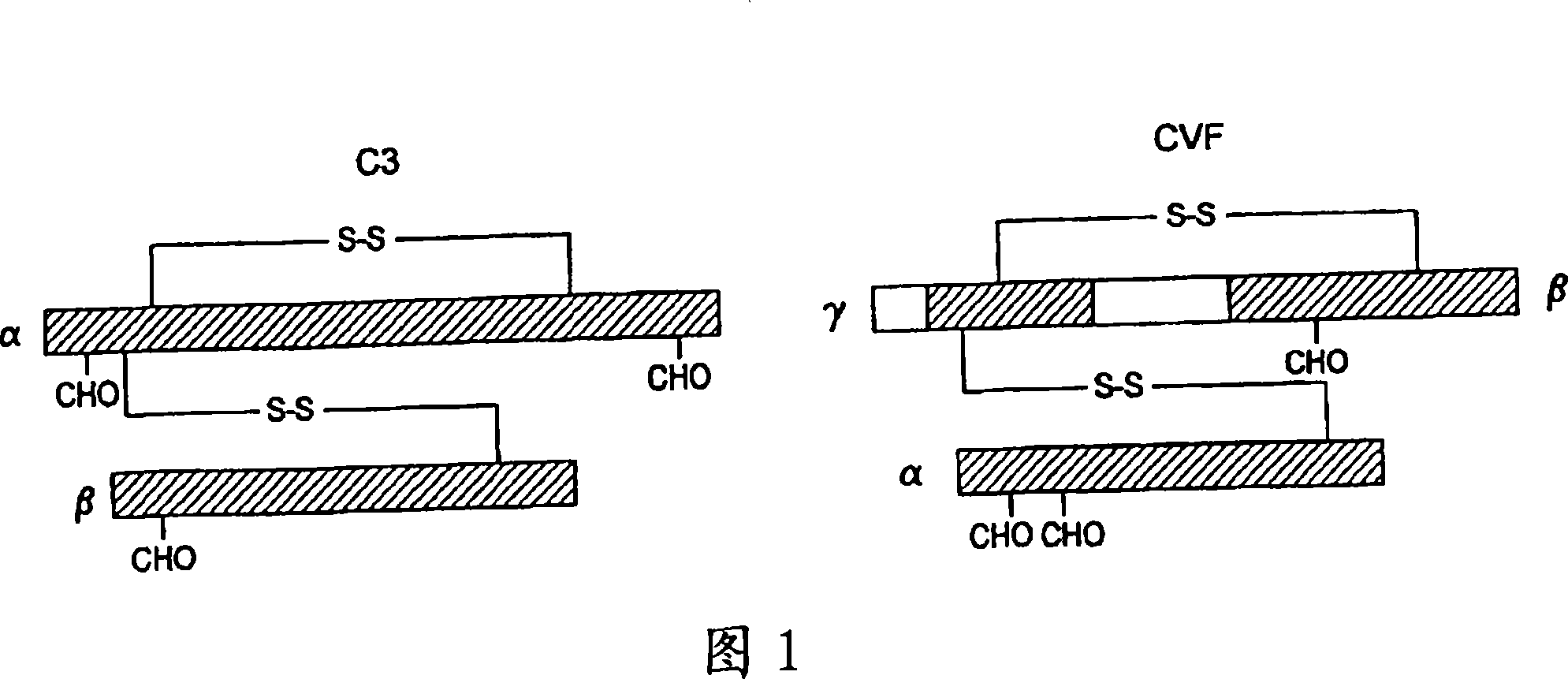
Human complement c3 derivatives with cobra venom factor-like function
A cobra venom factor and complement technology, applied in the field of chimeric derivatives of human complement C3, can solve problems such as serum complement activity failure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0083] Example 1: Production of human complement C3 / CVF hybrid protein
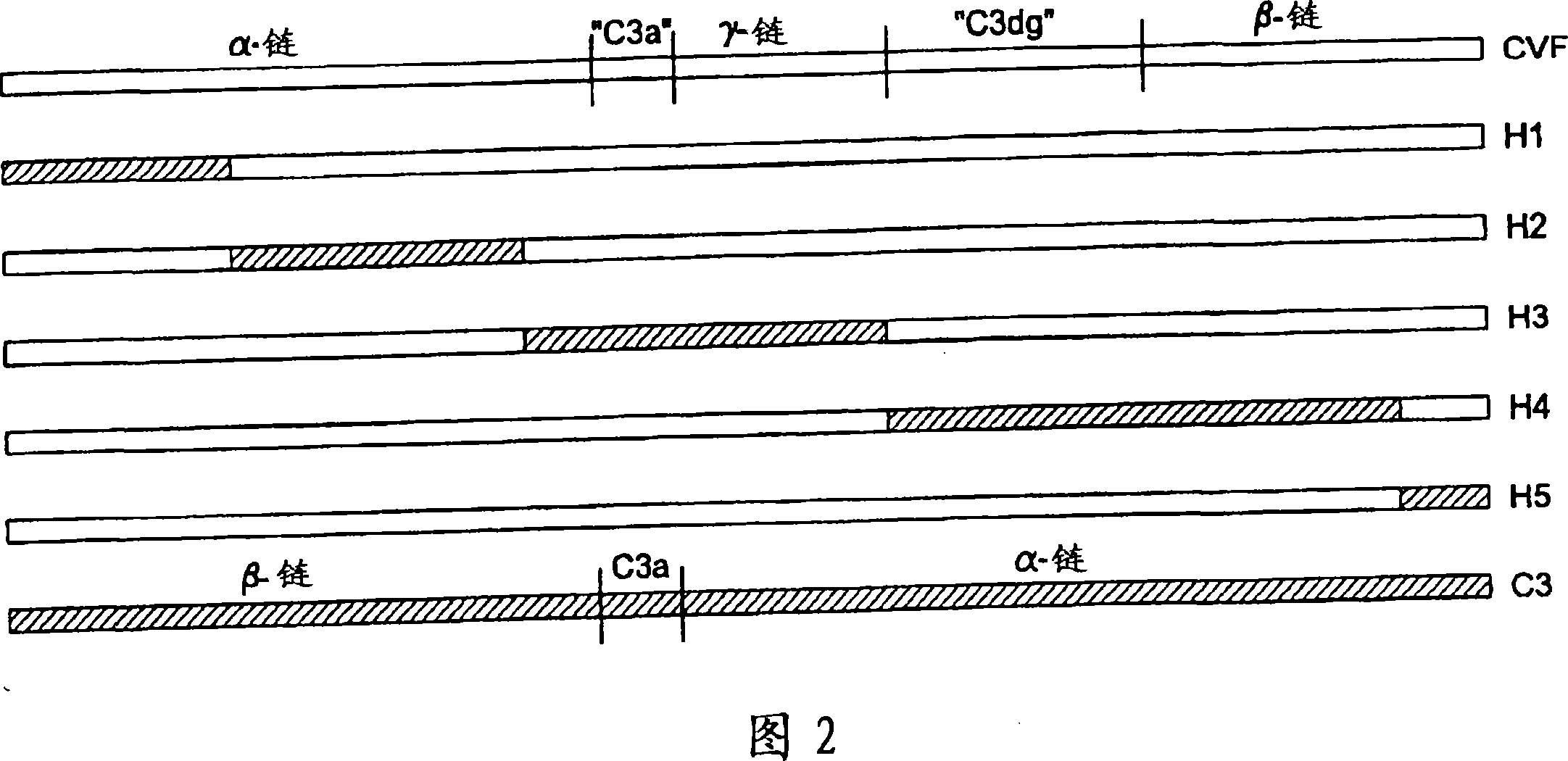
[0084] Replacing the human C3 sequence with a CVF sequence that represents important structural features of CVF-specific functions results in the generation of C3 derivatives with CVF-like functions. Accordingly, embodiments of the present invention relate to the generation of human C3 derivatives that exhibit complement-depleting CVF-specific functions by forming stable convertases for use as novel therapeutic agents that deplete complement in clinical diseases in which complement activation is part of the etiology. Since the structural changes of human C3 caused by the specific sequence of CVF are small, it is clear that the modified C3 molecules can show significantly reduced or even no immunogenicity.
[0085] The human C3 molecules in Table 1 were designed to contain specific CVF sequences in order to generate CVF-functional human C3 derivatives. Some embodiments of the invention provide for minor s...
Embodiment 2
[0098] Example 2: Expression of modified human C3 protein
[0099] The protein was produced in the Drosophila S2 cell system using the Drosophila Bip signal sequence to secrete the protein. Briefly, plasmid pMB / HC3-1550, pMB / HC3-1504, pMB / HC3-1496, pMB / HC3-1550 / 1617 and pMB / HC3-1348 were transfected into Drosophila S2 cells. S2 cells were transfected with a mixture of expression plasmids and pCoBlast in a 19:1 (w:w) ratio. After transfection, cells containing the two plasmids were selected with blasticidin (25 μg / ml). For expression, a 1 liter culture of transfected cells was grown in serum-free medium (Hi-Five plus L-glutamine) without blasticidin. When the cell density reaches 5×10 6 At 1 cell / ml, CuSO4 was added to a final concentration of 25 μM to induce recombinant protein production. Allow the cultures to express the recombinant protein for 4-5 days. The hybrid protein was then purified from the culture medium by combined ANX, Sephacryl H-300 and CM-FF chromatog...
Embodiment example 3
[0102] Embodiment 3: Activity test result of modified human complement C3 protein
[0103] The purified modified human C3 protein hybrids were subjected to various functional assays as follows.
[0104] Complement depletion
[0105] This test detects the ability of a protein to deplete complement in human (or other) serum. This test proceeds in two steps. In the first step, the protein of interest is usually diluted in buffer to expected concentration. A 10 microliter portion of the diluted protein was then mixed with undiluted serum. The mixture is incubated at 37°C for 3 hours, which allows the protein to activate complement by forming a C3 convertase. The subsequent formation of convertase activates C3 in serum. The amount of remaining complement activity is then determined by diluting the serum with antibody-sensitized sheep red blood cells that are readily lysed by complement when present in the serum and mixing with the serum. The reaction was allowed to proce...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com