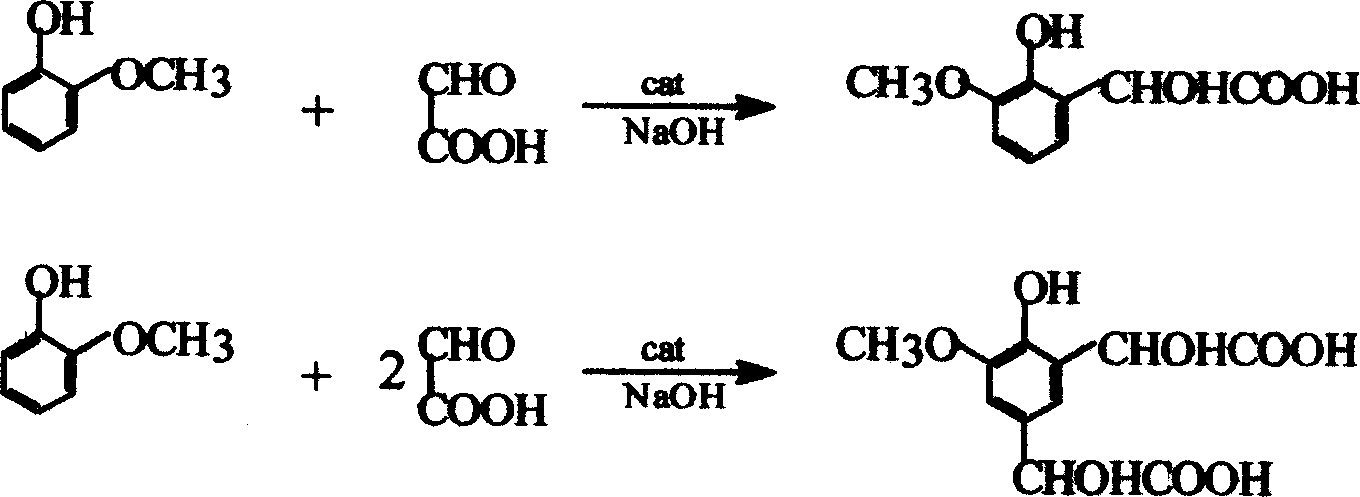
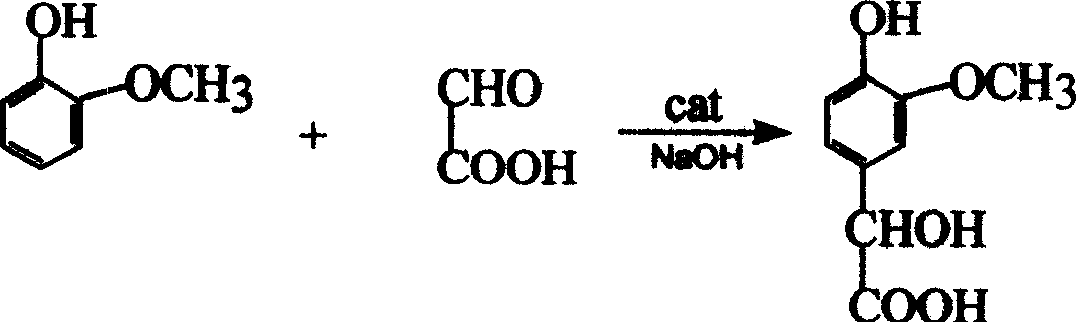Method of synthesizing 3-methoxy-4-dydroxymandelic acid or 3-ethoxy-4-dydroxymandelic acid by acetaldehyde acid method
A technology of hydroxymandelic acid and glyoxylic acid, applied in chemical instruments and methods, preparation of organic compounds, preparation of carboxylate, etc., can solve problems such as low yield and unsatisfactory yield, and achieve more efficient and mild reaction And the effect of stabilizing and reducing the generation of by-products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] (1) Weigh 37g of glyoxylic acid and add it to 57g of water, stir well and set aside.
[0024] (2) Weigh 18.8g of sodium hydroxide and add 140g of water to prepare a sodium hydroxide solution for later use.
[0025] (3) Weigh 27 g of methylguaiacol with a mass content of 99.4% for later use.
[0026] Weigh 110g of water and add it to the condensation kettle equipped with a constant temperature water bath, agitator and thermometer, start stirring, raise the temperature to 28°C, add 0.5g of catalyst A (tetramethylammonium hydroxide), and then add 17g of the above (2) to prepare A good aqueous sodium hydroxide solution, after the temperature is stable, drips the glyoxylic acid solution prepared by the above-mentioned (1) simultaneously through three pipelines respectively,
[0027] (2) The remaining aqueous sodium hydroxide solution and (3) methyl guaiacol were dropped after 3 hours, and then continued to stir and react for 1 hour under this condition. After the reaction,...
Embodiment 2
[0029] According to the operating steps of Example 1, catalyst B (tetramethylammonium chloride), catalyst C (tetramethylammonium bromide), catalyst A plus B (by mass, A: B=1: 1), Catalyst A plus C (by mass, A:C=1:1), catalyst B and C (by mass, B:C=1:1), catalyst A plus B plus C (by mass, A:B : C=1: 2: 2), all the other technological conditions remain unchanged with embodiment 1, and test result sees attached table.
Embodiment 3
[0031] According to the operating steps of embodiment 1, except changing the reaction time, all the other processing conditions are identical with embodiment 1, and test result sees the attached table below.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com