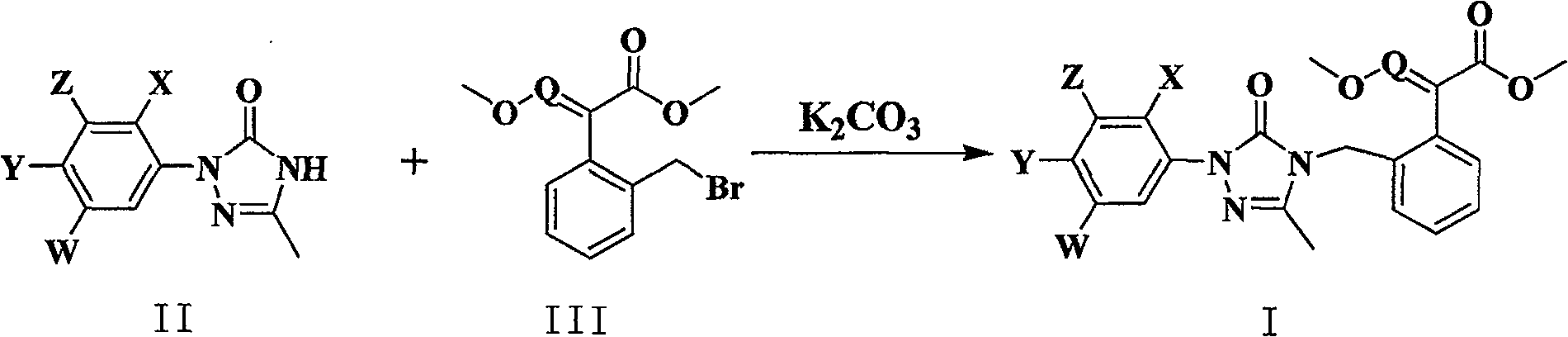
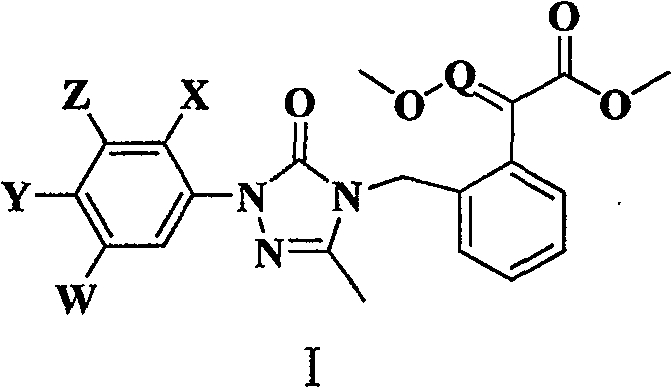
Synthesis of 4-substituted methoxy acrylate-1,2,4-triazolinones derivatives and herbicidal activity
A methoxyacrylate and triazolinone technology is applied in the field of synthesis and herbicidal activity of a class of 4-substituted methoxyacrylate-1,2,4-triazolinone derivatives, and can solve the problem of No problems such as reports have been reported, and the effect of significant herbicidal activity has been achieved
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] preparation of
[0027] Step A: Preparation of intermediate pyruvate-2-fluoro-4-chlorophenylhydrazone
[0028] Add 20 grams (0.137 moles) of 2-fluoro-4-chloro-aniline and 160 milliliters of concentrated hydrochloric acid to a 500 milliliter three-necked flask under nitrogen protection, cool to -9°C in an ice-salt bath, and slowly add 50 milliliters of 9.5 g (0.137 moles) of sodium nitrite in aqueous solution (about 30 minutes), after addition, stirred at -9°C-0°C for 1 hour. Then at this temperature, 68 milliliters of concentrated hydrochloric acid solution (about 40 minutes) containing 68.1 grams (0.3 moles) of tin chloride was added dropwise. 110 ml of water were added and 125 ml of an aqueous solution containing 12.2 g (0.137 mol) of pyruvic acid was added within 5 minutes. Continue to stir for 30 minutes, filter with suction, wash with water, and dry to obtain 27.7 g of the product, m.p.162-163°C. This process was repeated 3 more times.
[0029] Step B: Prepar...
Embodiment 2
[0032] preparation of
[0033] Step A: Preparation of intermediate 2-(2-fluoro-4chloro-5-nitrophenyl)-5-methyl-1,2,4-triazolin-3-one
[0034] Dissolve 0.05 mole of 2-(2-fluoro-4-chlorophenyl)-5-methyl-1,2,4-triazol-3-one in 100 ml of concentrated sulfuric acid, slowly add 68% nitric acid 4.5 gram, control the reaction temperature below 25°C, after the addition is complete, stir at 25°C for 1 hour, pour into ice water, filter, wash, and dry to obtain 9.5 grams of the product. This process was repeated 3 more times.
[0035] Step B: Preparation of intermediate 2-(2-fluoro-4chloro-5-aminophenyl)-5-methyl-1,2,4-triazolin-3-one
[0036] 0.01 mole of 2-(2-fluoro-4-chloro-5-nitrophenyl)-5-methyl-1,2,4-triazolin-3-one, 0.55 g of ammonium chloride and 2.24 g (0.04 mol) reduced iron powder was added to 25 milliliters of ethanol and 3 milliliters of water, heated and refluxed for 4-6 hours, cooled, then filtered with diatomaceous earth and washed with ethanol. The filtrate was conc...
Embodiment 3
[0051] preparation of
[0052] In a 250 ml single-necked flask, add 80 ml of N,N-dimethylformamide solution, 0.01 mole of 2-(2-fluoro-4 chlorophenyl)-5-methyl-1,2,4-tri Azolin-3-one, 2.1 g (0.015 mol l) of anhydrous potassium carbonate and 0.011 mol of (E)-methyl 2-(2-bromomethylphenyl)-3-methoxyacrylate. After stirring at room temperature for 24 hours, the reaction mixture was poured into ice water, and a large amount of white solid was precipitated, left to stand, filtered with suction, dried, and column chromatography (acetone:petroleum ether=1:6v / v) gave the product, white solid, yield 43%; m.p. 113-115°C.
[0053] Elemental Analysis: Calculated C% 58.41 H% 4.43 N% 9.73
[0054] Measured value C% 58.09 H% 4.28 N% 9.74
[0055] 1 H NMR (400MHz, CDCl 3 , δ / ppm): δ1.93 (s, 3H, CH 3 ), 3.64 (s, 3H, CO 2 CH 3 ), 3.87 (s, 3H, OCH 3 ), 4.66, 4.86 (ss, 2H, CH 2 ), 7.19-7.57 (m, 8H, ArH, CH=);
[0056] MS (EI 70eV) m / z (%): 432 ([M] + , 56), 399 (67.28), 205 (...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com