Galanthamine hydrobromide composition and its preparing method
A technology of galantamine hydrobromide and a composition, which is applied in directions such as drug combination, active ingredients of heterocyclic compounds, pill delivery, etc., can solve the problems of inconvenience of patients, short action time of preparations, etc., and achieve the effect of convenience for patients
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
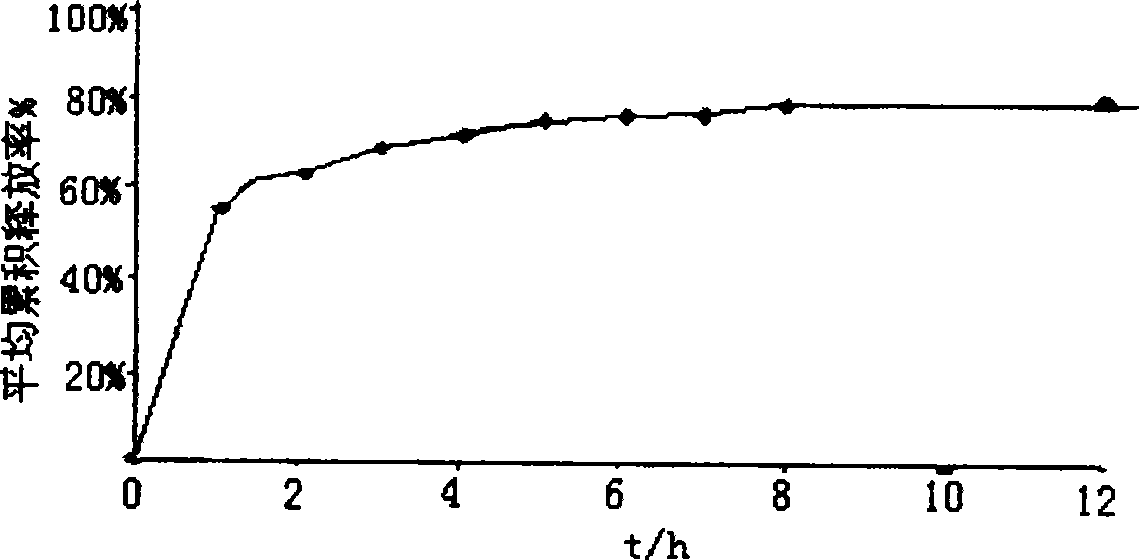
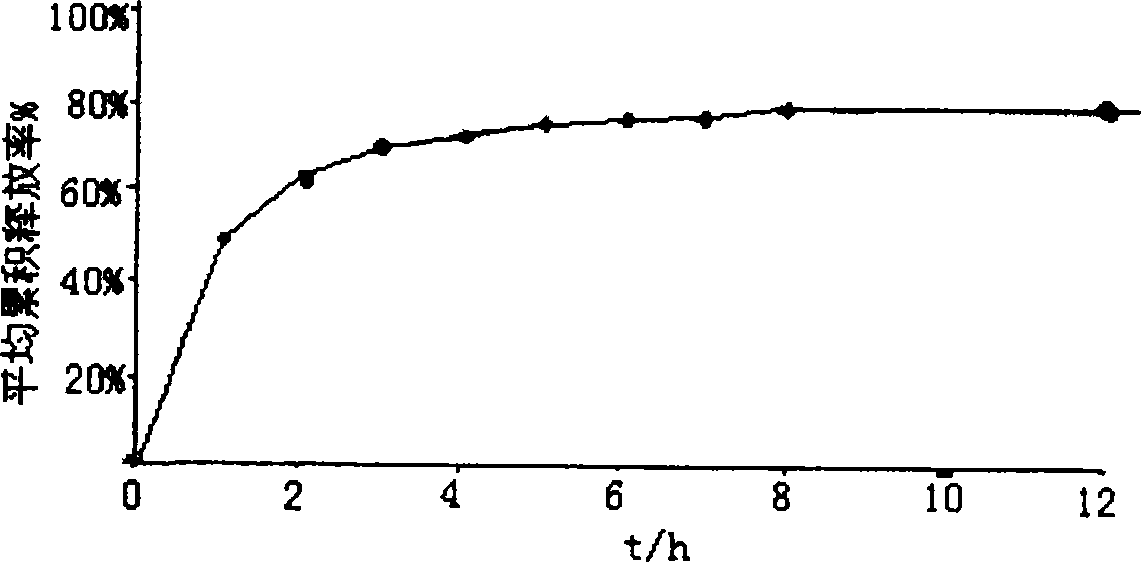
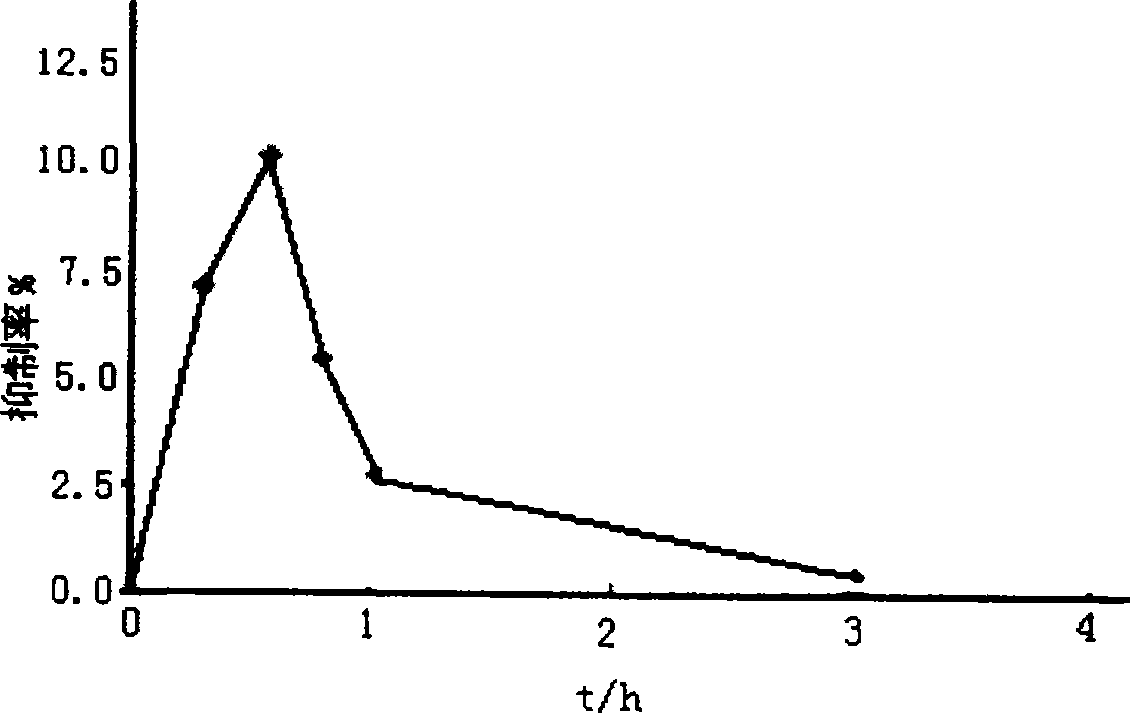
[0038] Suspend 5 g of 200-mesh sulfonic acid cation exchange resin in 100 ml of deionized water, add 2.5 g of galantamine hydrobromide, heat and stir at 60°C for 2 hours, filter, and dry to obtain galantamine hydrobromide Sustained release composition.
Embodiment 2
[0040] Suspend 20 g of 200-mesh sulfonic acid cation exchange resin in 60 ml of deionized water, add 0.5 g of galantamine hydrobromide, heat and stir at 60°C for 2 hours, filter, and dry to obtain galantamine hydrobromide Sustained release composition.
Embodiment 3
[0042] Add 5 g of the product of Example 1 into 100 ml of a cyclohexane solution containing 0.5 g of ethyl cellulose, heat and stir to reflux temperature, keep it warm for 1 hour, cool to 20 ° C, filter, and dry to obtain hydrogen cellulose coated with ethyl cellulose. Galantamine Bromate Sustained Release Preparation. Components and parts by weight are as follows:
[0043] Sulfonic acid cation exchange resin 5g, galantamine hydrobromide 2.5g, ethyl cellulose 0.5g.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com