New type quick oral disintegration tablet of Subing and preparation method
A technology for oral fast disintegrating tablets and borneol, which is applied in the field of Su Bing oral fast disintegrating tablets and its preparation, can solve the problems of difficult tablet preparation, inconvenience for severe patients to take, unstable drug content, etc., so as to facilitate first aid and prevention and ensure curative effect. and storage period content, the effect of relieving chest tightness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
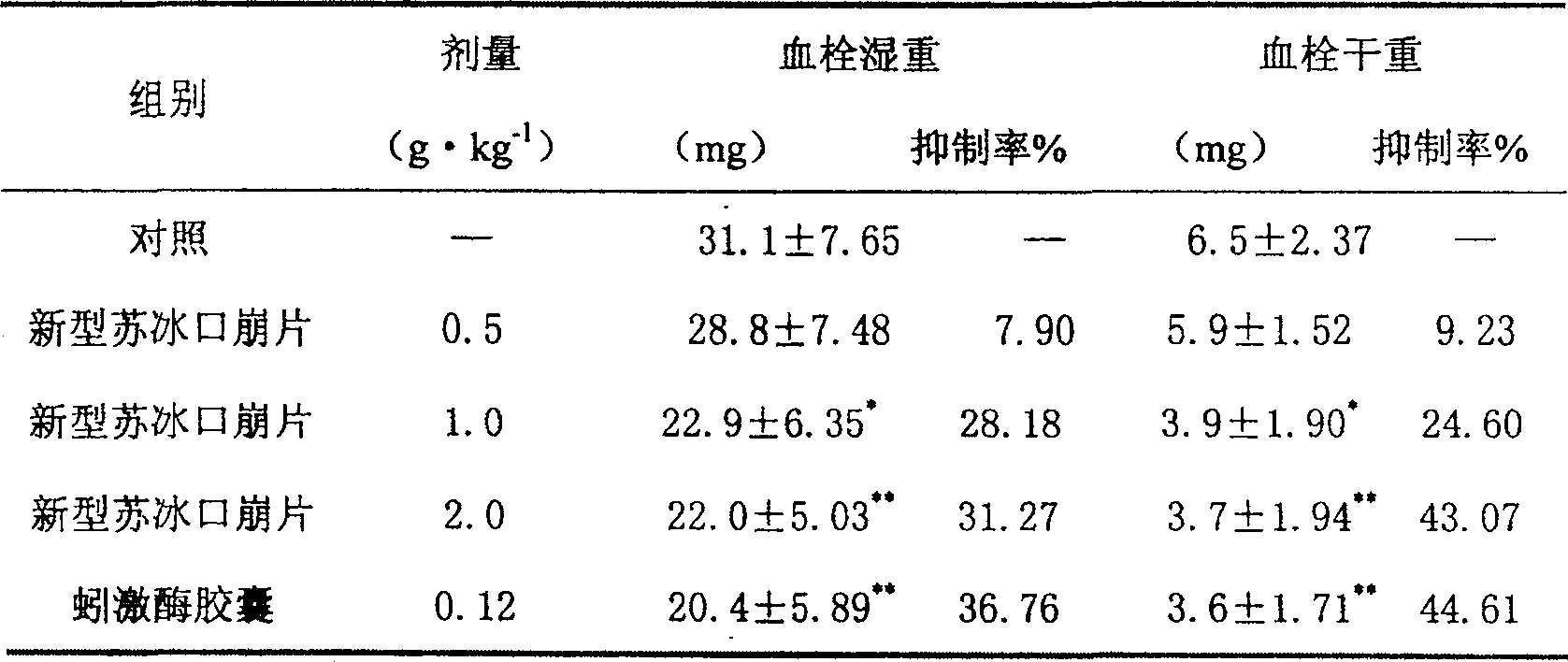
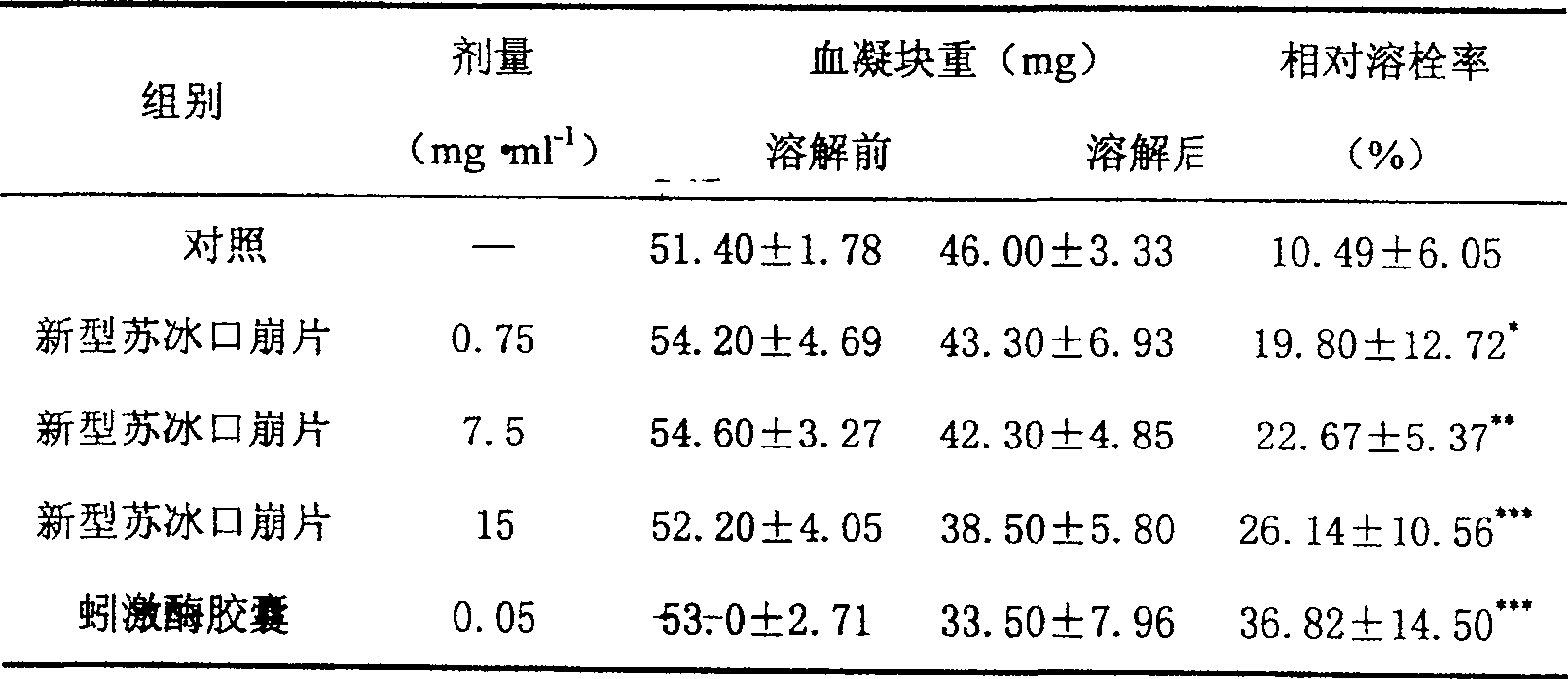
Image
Examples
Embodiment 1
[0062] Selection and handling of medicinal ingredients
[0063] ·Medicinal ingredients: styrax and borneol.
[0064] · Handling of medicinal ingredients:
[0065] First of all, the above-mentioned volatile medicinal materials are all pre-treated according to the conventional methods stipulated in the Pharmacopoeia;
[0066] Then, the medicinal active ingredient is made into a solid dispersion, and the method is:
[0067] Take 10 g of Styrax volatile oil and add it into 50 ml of ethanol solution containing 6% Eudragit E-100 to prepare an organic phase. Take another 1000ml of water, add 30g of polyethylene glycol to disperse, and form a water phase; under the condition of stirring the water phase, drop the organic phase into the water phase at a constant speed, crosslink and solidify while stirring, and the time is 2 The medicinal styrax-coated drug-loaded microcapsules were obtained within 1 hour; the organic solvent was extracted with 15 liters of water, and then filtered a...
Embodiment 2
[0086] Take 30g of sodium alginate and add it to 100ml of water, then add 10g of styrax volatile oil and stir evenly, then add it dropwise to the chitosan glacial acetic acid solution of calcium chloride (the preparation method of this solution: take 1000ml of water, add 20ml of glacial acetic acid, 5g Chitosan and 15g calcium chloride) were stirred for 1 hour to obtain the styrax-coated drug-loaded microcapsules. After filtering and drying, the easy-flowing coated storax drug-loaded microcapsules capable of releasing medicinal components are obtained.
[0087] Tablet formulation (by weight) components are:
[0088] Medicinal ingredients Styrax loaded with medicine 4.40g
[0089] Drug-loaded borneol 5.60g
[0090] Microcrystalline Cellulose 19.0g
[0091] Mannitol 14.4g
[0092] Sodium carboxymethyl starch 15.0g
[0093] Mint 0.25g
[0094] Stevioside 0.05g
[0095] Magnesium stearate 0.65g
[0096] Micronized silica gel 0.65g
[0097] A tot...
Embodiment 3
[0102] The selection and processing of medicinal ingredients are the same as in Example 1.
[0103] The formula (by weight) components are:
[0104] Medicinal ingredients Styrax loaded with medicine 4.40g
[0105] Drug-loaded borneol 5.60g
[0106] Microcrystalline Cellulose 21.00g
[0107] Mannitol 15.60g
[0108] Cross-linked polyvinylpyrrolidone 12.00g
[0109] Stevioside 0.05g
[0110] Mint 0.25g
[0111] Magnesium stearate 0.55g
[0112] Micronized silica gel 0.55g
[0113] A total of 1000 pieces were made
[0114] The specific preparation method is:
[0115] Mix the stevioside crushed through the 80-mesh sieve with the drug-loaded styrax and borneol according to the prescription amount, and pass the cross-linked polyvinylpyrrolidone, microcrystalline cellulose, mannitol, micronized silica gel, and magnesium stearate through the 80-mesh sieve respectively Mix evenly according to the prescription amount, then mix evenly with the medicinal i...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com