Fusion protein of chemoattracting small peptide and dual specific antibodies
A bispecific antibody and fusion protein technology, applied in the direction of anti-animal/human immunoglobulin, hybrid immunoglobulin, anti-receptor/cell surface antigen/cell surface determinant immunoglobulin, etc., can solve the problem of effector cells Limited quantity, difficulty in getting the most out of the role, etc., to achieve the effect of great application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Example 1: Construction of vector pTCMBO.
[0044] (1) Treatment of vector pTMF.
[0045] The construction process of PTMFMH. Firstly, the vector pTMF (the vector pTMF is derived from the vector pET28a(+) (Novagen Company), which is constructed by replacing the multiple cloning site of the vector pET28a(+) with a segment of multiple cloning sites synthesized by our company, see reference 30) with Digest with restriction endonuclease BamHI (Bao Bioengineering (Dalian) Co., Ltd., the same below), as follows: take 18 μl of vector pTMF, 1.5 μl of BamHI, 3 μl of K buffer, and use ddH 2 O to make up the volume to 30 μl, and enzymatic hydrolysis reaction in 37°C water bath for 4 hours. Purify and recover the enzyme-digested DNA according to the instructions of the Small Volume Gel Recovery Kit (product of Huashun Biotechnology Co., Ltd., the same below). The recovered product was digested with Bpu1102I, specifically as follows: pTMF 16 μl, Bpu1102I 1.5 μl (purchased from Tr...
Embodiment 2
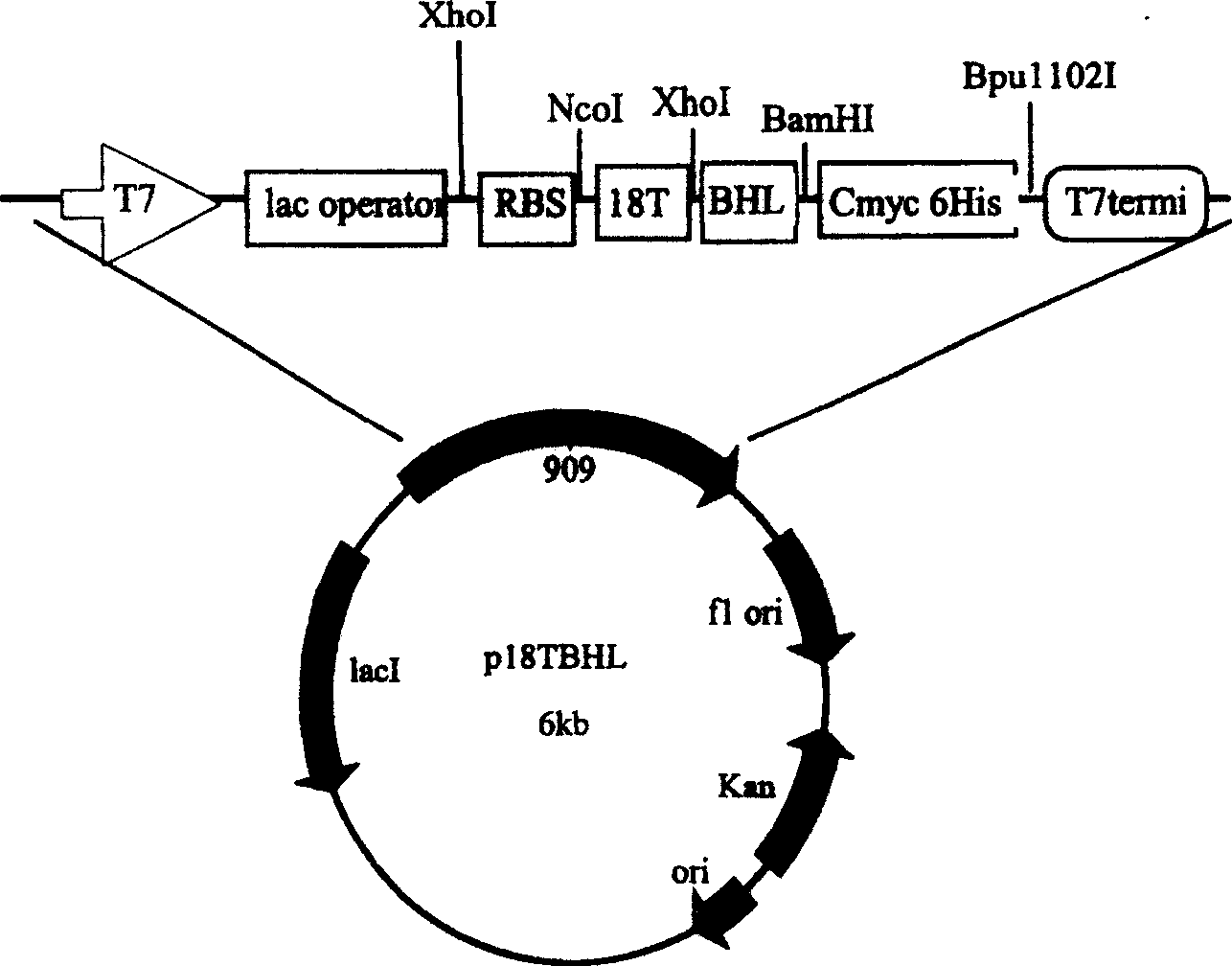
[0065] Example 2: Construction of vector p18TBHL
[0066] Preparation of chemokine 18 peptide
[0067] According to the sequence of SLC, design and synthesize two complementary chains that generate N-terminal 18 peptides
[0068] etup 5'agcgatggtgcacaggattgctgcctgaaatatagccagcgtaaaattccg3'
[0069] etdown 5'ctcgagagaaccaccaccaccagaaccaccaccacccggaattttacgctggctata 3' two complementary strands were amplified by PCR to obtain a complementary double strand containing restriction site XhoI at the 3' end. For specific operations, refer to the Molecular Cloning Guide. The PCR product was recovered with a mini-gel recovery kit. The recovered PCR product was subjected to XhoI single enzyme digestion, and the enzyme digestion reaction was referred to the product manual, and the enzyme-digested fragment was purified and recovered with a small gel recovery kit.
[0070] Processing of vector pTCMBO
[0071] The vector pTCMBO was first digested with NcoI (purchased from Treasure Bioengi...
Embodiment 3
[0079] Example 3: Low temperature-induced intracellular soluble expression of p18TBHL
[0080] (1) Transform p18TBHL into Escherichia coli BL21(DE3)
[0081] According to the method for preparing competent cells described in (4) of Example 1, competent cells of Escherichia coli BL21(DE3) (Invitrogen Company) were prepared. The plasmid p18TBHL was extracted according to the instructions of the plasmid extraction kit (Shanghai Huashun Company), and the transformation experiment was carried out according to (5) of Example 1.
[0082] (2) Low temperature induced expression
[0083] Coat BL21(DE3) containing p18TBHL on LB-K plate, culture overnight at 37°C, then pick a single clone, inoculate it in 5ml LB-K liquid medium, and culture it overnight on a shaker at 37°C in a large test tube (200 rpm / minute). The next day, take the overnight culture and transfer it to 250ml LB-K liquid medium at a ratio of 1 / 100, and continue to culture on a shaker at 37°C (200 rpm) to A 600 =0.6, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com