Production technique of N,N'-dicyclo hexylcar bodiimide
A technology of dicyclohexylcarbodiimide and dicyclohexylthiourea, applied in the field of polypeptide dehydration condensing agent N, can solve problems such as difficult desulfurization, and achieve the effect of reducing production cost and improving product quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
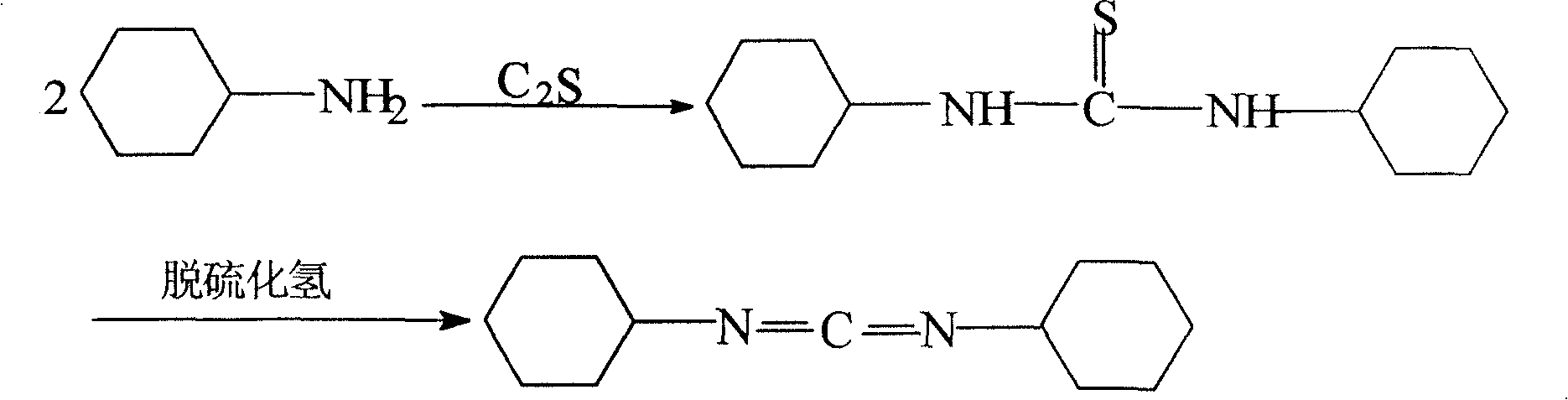
[0032] Add 30g of cyclohexylamine to 200ml of xylene, stir and add 10g of carbon disulfide dropwise, control the temperature at 50°C, reflux for 3 hours, heat filter, filter out the alkali sulfide, cool down the crystal and suction filter, dry to obtain white crystal N,N-di Cyclohexylurea, yield 98%.
[0033] Add 30 g of N, N-dicyclohexylurea to 300 ml of benzene, add 1 g of catalyst (TEBA), stir and heat, add dropwise 100 ml of sodium hypochlorite solution (concentration 30%) at 70 ° C, stir for 2 hours, extract with water, and then use the same The sodium hypochlorite and catalyzer carry out secondary oxidation, and step is the same as above.
[0034] Add 20 g of the by-product sodium sulfide in the first step to the extract, under alkaline conditions, control the pH value to 9, stir and react for 2 hours, filter and extract with water separation, remove sulfur, and carry out distillation and rectification of the organic phase to obtain the product. The yield is 92%, and th...
Embodiment 2
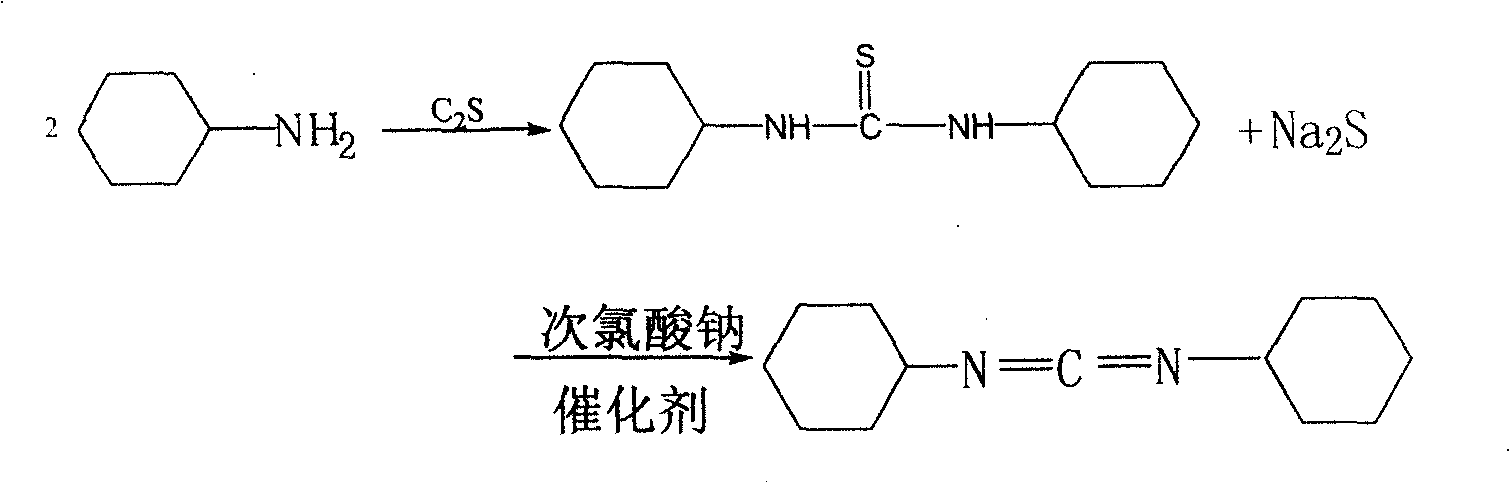
[0036] Add 80 kg of cyclohexylamine to 400 kg of xylene, stir and add 40 kg of carbon disulfide dropwise, control the reaction temperature at 60° C., reflux for 3 hours, heat filter, filter out alkali sulfide (for standby), cool down the crystal and suction filter, and dry to obtain white crystal N. N-Dicyclohexylurea.
[0037] Add N,N-dicyclohexyl urea 700kg in 500kg benzene. Add 30 kg of catalyst polyethylene glycol, stir and heat, add dropwise 800 kg of sodium hypochlorite solution (concentration 30%) at 70 ° C, stir for 2 hours, and extract with water. Then use 800 kg of sodium hypochlorite solution (concentration 30%) and 3 kg of polyethylene glycol Secondary oxidation, the steps are the same as above.
[0038] Add 200 kg of the by-product sodium sulfide in the first step into the extract (organic phase), under alkaline conditions, control the pH value to 8, stir and react for 2 hours, filter and extract with water separation, remove sulfur, and carry out distillation an...
Embodiment 3
[0040] With embodiment 1, difference is that the consumption of catalyst TEBA is 1.5kg. The product yield is 93%, and the product purity is above 99.5%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com