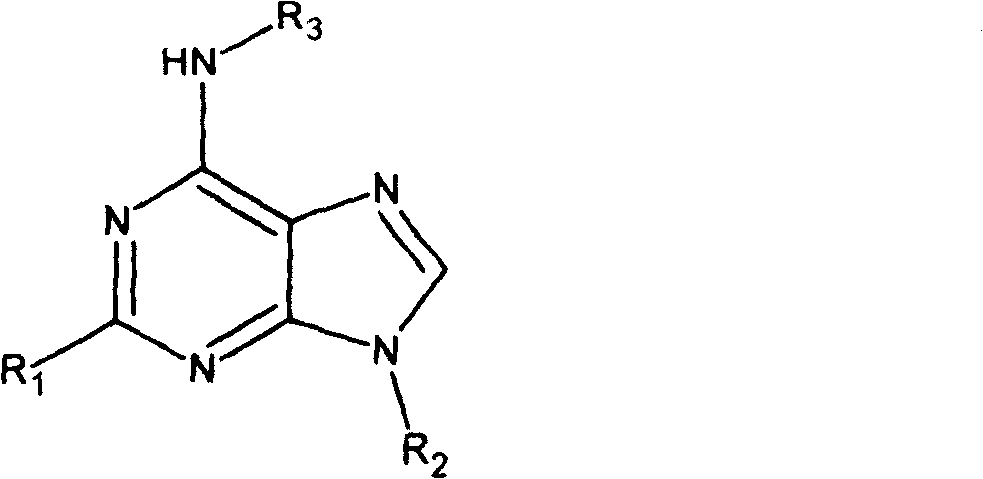
Adenine kind derivative and its synthesis method
A technology for adenine and derivatives, applied in the field of adenine derivatives and their preparation, can solve problems such as hazards, difficult sources of raw materials, low biological activity, etc., to improve cytokinin activity, facilitate industrial implementation, and improve anti-inflammatory activity Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] N 9 Synthesis of -(2-boronic acid) benzyl adenine:
[0037]
[0038] Add 270mg (2mmol) of adenine and 300mg (2.15mmol) of anhydrous potassium carbonate to 5ml of freshly steamed DMF to form a suspension, and heat the suspension to 120°C in an oil bath with stirring. Within 60 minutes, 5ml Dissolve 515.5 mg (2.4 mmol) of 2-bromomethylphenylboronic acid in DMF solution, dropwise added to the mixture, and reflux for 14 hours. As the reaction progresses, the color of the suspension gradually becomes darker, and finally it is earthy brown. Filtrate while hot, wash with hot anhydrous DMF 10ml fractions, combine the filtrates, evaporate the DMF under reduced pressure at 45°C, dissolve the concentrate with a small amount of methanol, and separate the product by thin-layer chromatography with a yield of 35.0% (developing agent is dichloro methane:methanol=8:1, volume ratio). The physical property data are shown in Table 1, IR, H 1 See Table 2 for NMR and MS data.
Embodiment 2
[0040] N 9 Synthesis of -(3-boronic acid) benzyl adenine:
[0041]
[0042] Add 270mg (2mmol) of adenine and 300mg (2.15mmol) of anhydrous potassium carbonate to 5ml of freshly steamed DMF to form a suspension, and heat the suspension to 120°C in an oil bath with stirring. Within 60 minutes, 5ml Dissolve 515.5 mg (2.4 mmol) of 3-bromomethylphenylboronic acid in DMF solution, dropwise added to the mixture, and reflux for 14 hours. As the reaction progressed, the color of the suspension gradually became darker, and finally it was earthy brown. Filtrate while hot, wash with hot anhydrous DMF 10ml fractions, combine the filtrates, distill off the DMF under reduced pressure at 45°C, dissolve the concentrate with a small amount of methanol, and separate the product by thin-layer chromatography with a yield of 42.5% (developing agent is dichloro methane:methanol=8:1, volume ratio).
[0043] The physical property data are shown in Table 1, IR, H 1 See Table 2 for NMR and MS data...
Embodiment 3
[0045] N 9 Synthesis of -(4-boronic acid)benzyl adenine
[0046]
[0047] Add 270mg (2mmol) of adenine and 300mg (2.15mmol) of anhydrous potassium carbonate to 5ml of freshly distilled DMF to form a suspension, and heat the suspension to 120°C with stirring. Within 60 minutes, dissolve 5ml of 515.5mg (2.4mmol) DMF solution of 4-bromomethylphenylboronic acid was added dropwise to the mixture, and refluxed for 14 hours. As the reaction progressed, the color of the suspension gradually became darker, and finally it was earthy brown. A large number of precipitates appeared, filtered while hot, washed with hot anhydrous DMF 10ml fractions, finally the precipitate was washed with water to obtain a white substance, dried in a vacuum oven for 1 hour, and recrystallized with methanol to obtain a white granular substance, the yield was : 46.1%.
[0048] The physical property data are shown in Table 1, IR, H 1 See Table 2 for NMR and MS data.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com