Use of CM101 in preparing medicine
A technology of 1.CM101, application, applied in the direction of drug combination, preservation and application of human or animal body, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0040] A method for the preparation of GBS toxin is provided in US Patent 5,010,062. Preferably, however, CM101 is purified as taught in International Application PCT / US97 / 17535, incorporated herein by reference.
[0041] The starting material for isolating CM101 used in the method of the present invention can be obtained by culturing strains of Group B β-hemolytic Streptococcus that have recently infected or are capable of infecting newborns. Isolates of this strain can be obtained from the blood or cerebrospinal fluid of infected neonates.
[0042] The GBS toxin used in the present invention is limited to any ingredient or component isolated from native or lysed GBS bacteria, or obtained from the culture supernatant of lysed and / or autoclaved GBS bacteria, and which is induced to breathe in sheep Biological activity confirmed by distress test (Hellerqvist, C.G. et al., Studies on group B beta-hemolytic streptococci I. Isolation and partial identification of extracellular to...
Embodiment 1
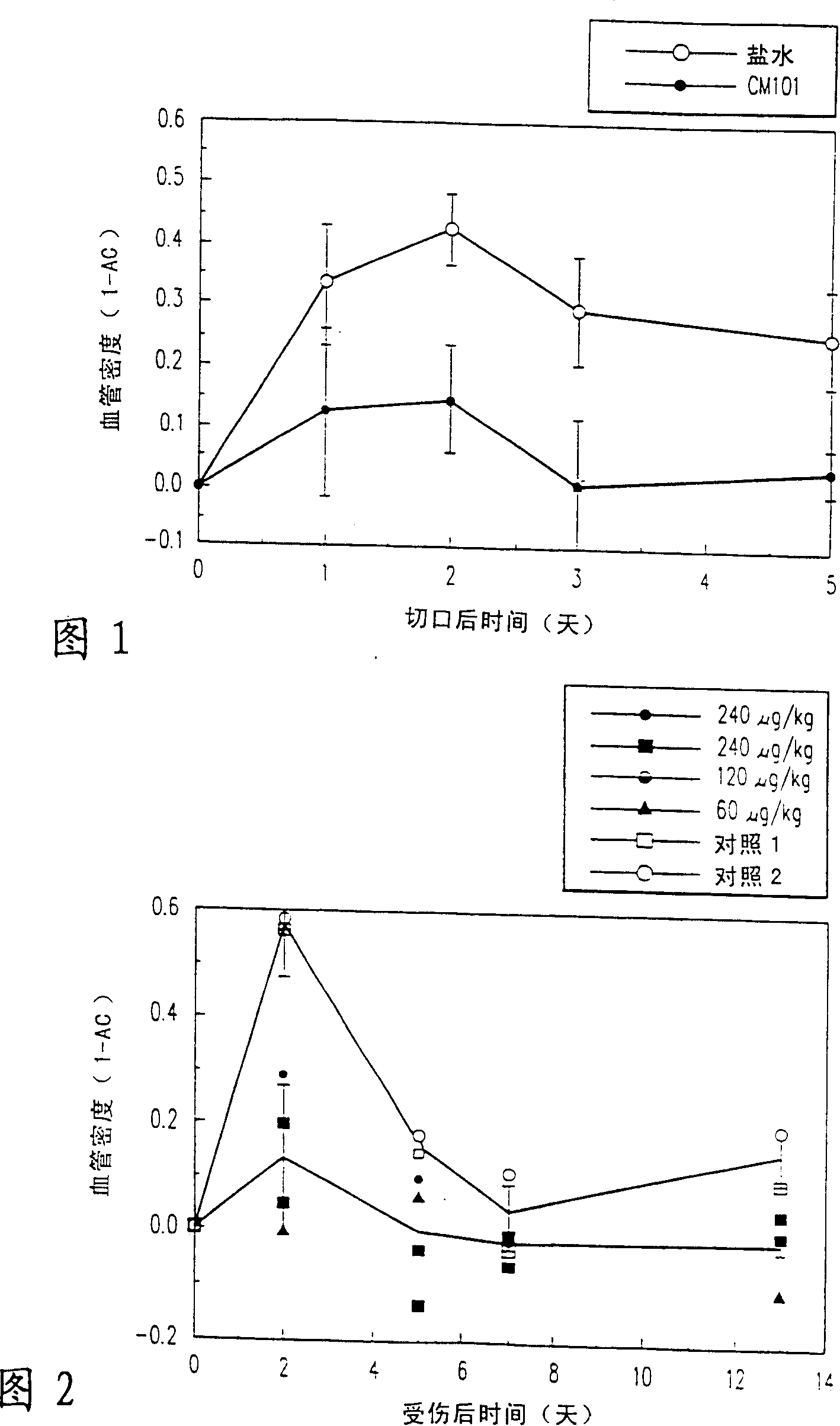
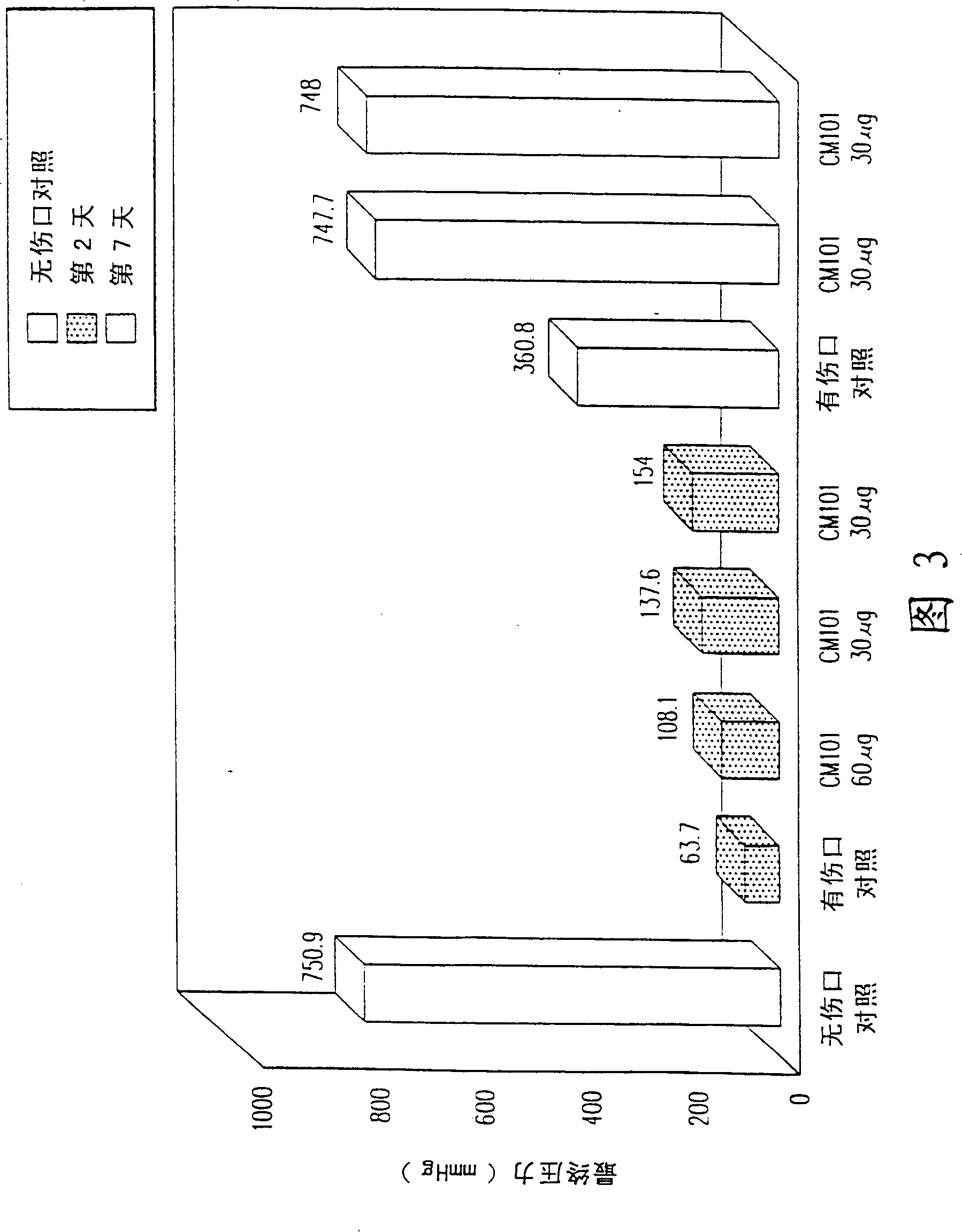
[0060] Example 1: CM101 promotes wound healing in tumor-free subjects.
[0061] The effect of CM101 on wound healing was determined in tumor-free CD-1 nude mice. The wound was made with fine surgical scissors and consisted of a 4 mm thick skin incision. with a sterile adhesive bandage (Tegadem Tm , USA) to cover each wound.
[0062] On day 0 of the experiment, mice were intravenously administered 240 μg / kg CM101 or saline and wounded. Neovascularization at each wound site was assessed using magnetic resonance microimaging (MRI), which was performed on days 0, 1, 2, 3, and 5.
[0063] MRI experiments were performed on a horizontal 4.7 T biopsy spectrometer (Bruker, Germany) using a 2 cm surface radiofrequency coil. Gradient echo images (slice thickness 0.5 mm, TR 100 ms, 256×256 pixels, 110 μm plane resolution) were acquired with echo times of 10.5 and 20 ms. Growth in the capillary bed was reflected by a decrease in mean intensity in the 1 mm area around the incision. An...
Embodiment 2
[0065] Example 2: CM101 Promotes Wound Healing in Tumor Subjects.
[0066] The effect of CM101 on wound neovascularization was examined using mice implanted with glioma spheroids. A single C6 glioma spheroid (approximately 800 μm in diameter) was implanted subcutaneously in the lower dorsum of each male CD-1 nude mouse (Abramovitch R., Meir G. and Neeman M., Neovascularization induced by implanted C6 nerve Growth of Glioma Multicellular Spheroids: Magnetic Resonance Microimaging, Cancer Research, 55: 1956-1962 (1995)).
[0067] Eight days later, on day 0 of the experiment, mice were anesthetized by intraperitoneal injection of 75 μg / g ketamine and 3 μg / g xylazine. Mice were injected with saline containing 0, 60, 120 or 240 μg / kg CM101 via the tail vein. Wound site neovascularization was assessed using Magnetic Resonance Imaging (MRI) as described in Example 1. Each anesthetized mouse was placed in the supine position in the MRI equipment and the tumor was located in the cen...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com