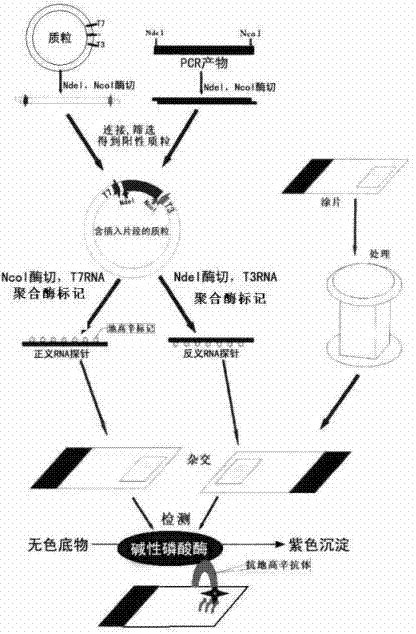
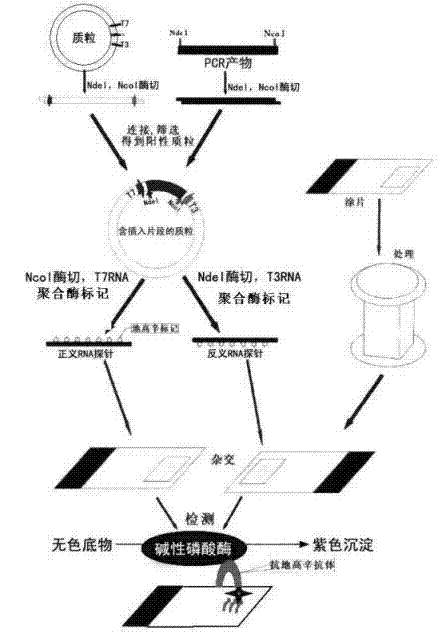
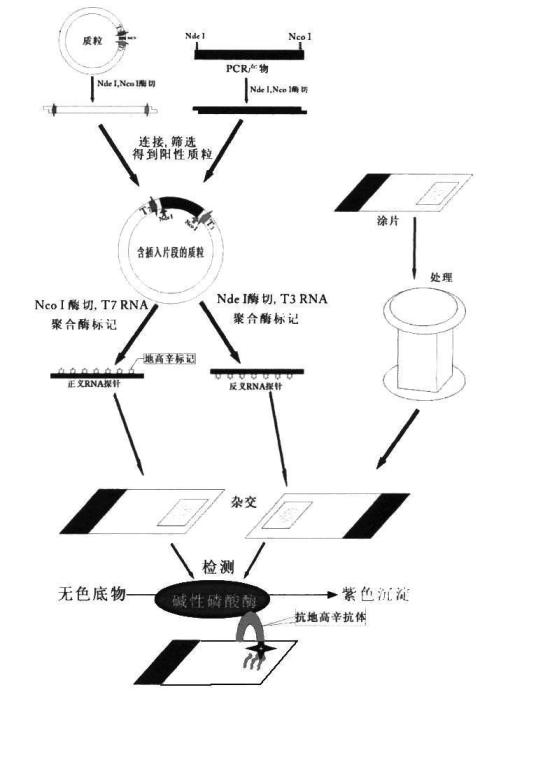
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
36results about How to "Implement early treatment" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
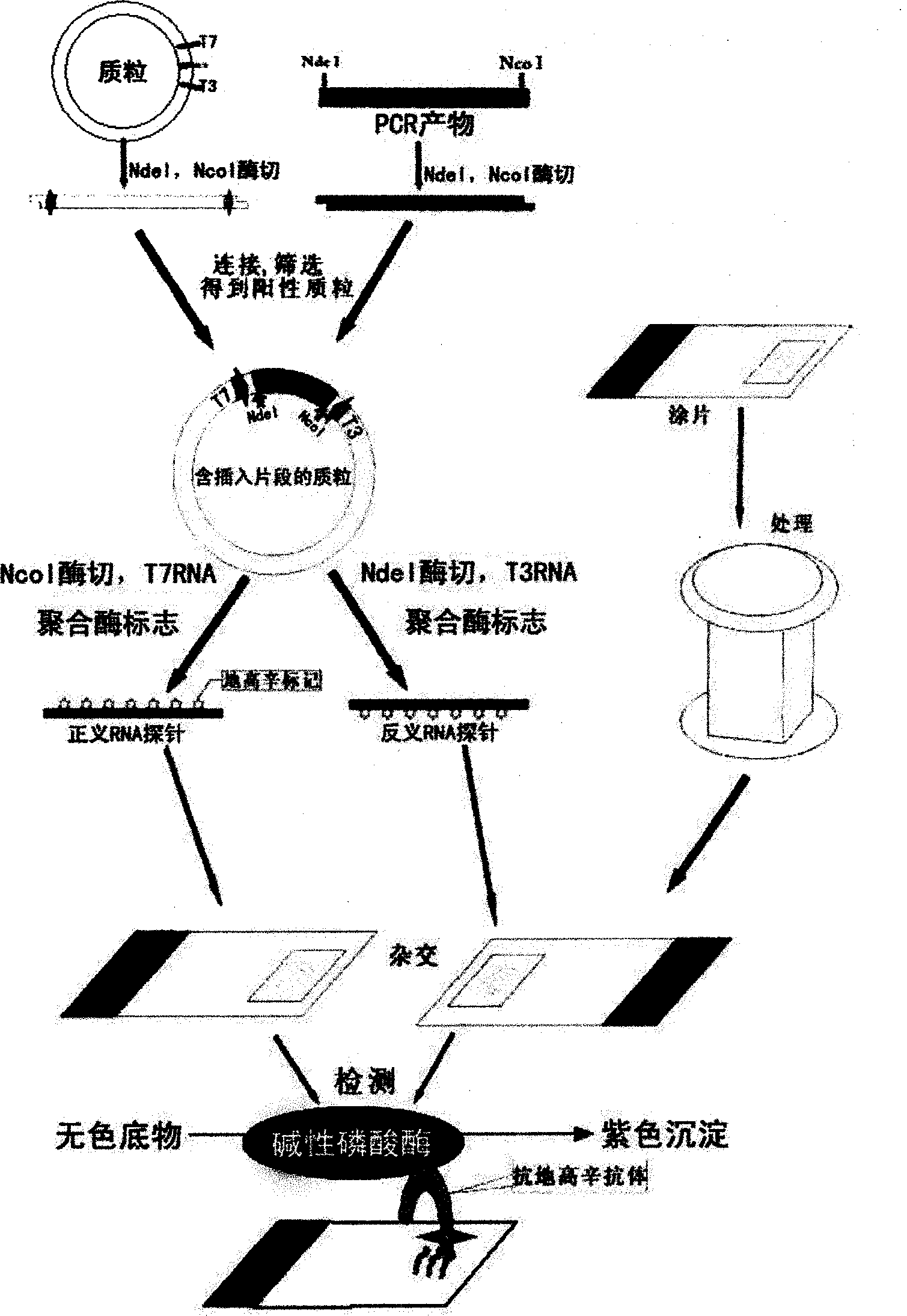
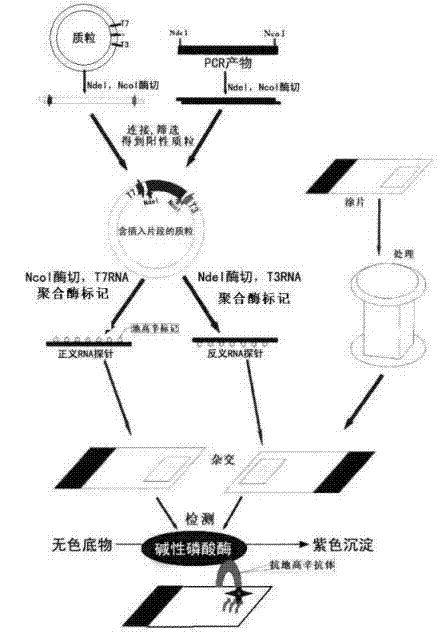
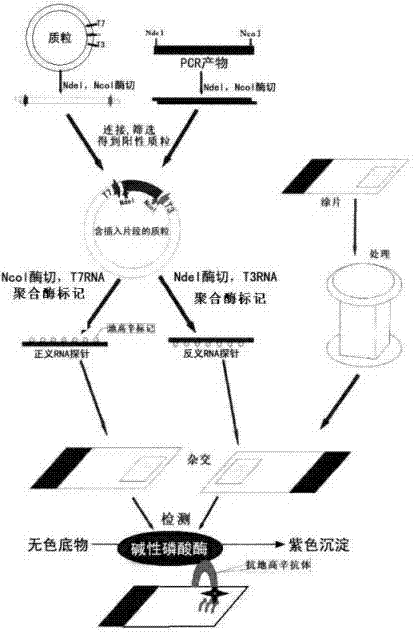
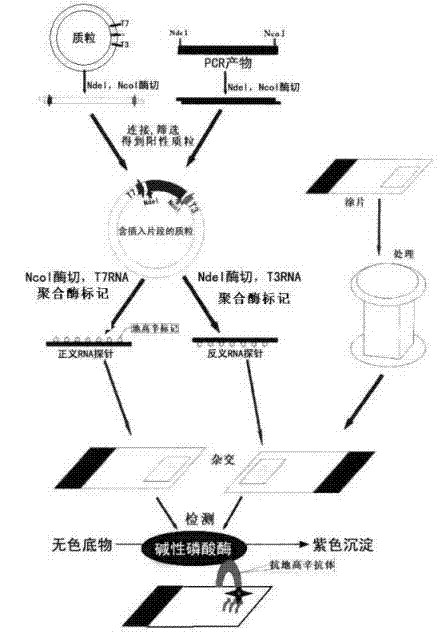
Kit for broad spectrum cancer hybridization in situ, detection method and application thereof
InactiveCN101363046AHigh sensitivityStrong specificityMicrobiological testing/measurementHybridization probeProstate cancer
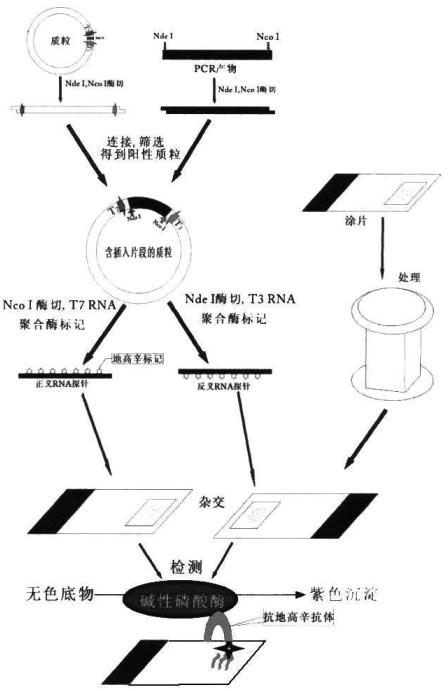
The invention relates to a kit for detecting in situ hybridization of broad-spectrum cancer, comprising a hybridization probe and a label, and the sequence of the hybridization probe is shown in SEQ ID NO.1. The invention also provides a method for detecting in situ hybridization of TROP-2 gene. In addition, the invention provides the application of the kit in the preparation of medicine for detecting cancers and diseases, and the cancers includes lung cancer, gastric cancer, breast cancer, carcinoma of colon, prostatic cancer, uterine cancer, cancer of pancreas, carcinoma of urinary bladder, pituitary cancer or glioma. The kit provided by the invention has the characteristics of high sensitivity and better particularity. The detecting method of the invention is convenient and simple in operation, and can be generally used and popularized in the hospitals which are above county level.
Owner:NATUREGEN BIOTECH SHANGHAI
In-situ hybridization detection kit for Spink1 genes and detection method and application thereof
InactiveCN101988110AHigh sensitivityStrong specificityMicrobiological testing/measurementSerine peptidaseHybridization probe
The invention relates to an in-situ hybridization detection kit for Spink1 (serine peptidase inhibitor, Kazal type 1) genes, which comprises a hybridization probe and a marker, wherein the sequence of the hybridization probe is expressed as SEQ ID No.1. The invention also provides an in-situ hybridization detection method for the Spink1 genes. In addition, the invention also provides application of the kit in preparation of a medicament for detecting prostatic cancer diseases. The kit provided by the invention has the advantages of high sensitivity and strong specificity. The detection method is convenient and simple to operate, and can be universally used and popularized in hospitals of above district level.
Owner:SUZHOU FUYING GENE TECH
In-situ hybridization assay kit and detection method for MTDH gene and application of kit
InactiveCN101993935AHigh sensitivityStrong specificityMicrobiological testing/measurementIn situ hybridisationHybridization probe
The invention relates to an in-situ hybridization assay kit for MTDH gene. The in-situ hybridization assay kit comprises a hybridization probe and a marker, wherein the sequence of the hybridization probe is shown as SEQ ID NO. 1. The invention also provides an in-situ hybridization detection method for the MTDH gene. In addition, the invention also provides application of the kit for preparing medicaments for detecting breast cancer. The invention has the advantages that: the provided kit has the characteristics of high sensitivity and high specificity; and the detection method is convenient and easy to operate and can be commonly used and popularized in hospitals of district-level and above.
Owner:SUZHOU FUYING GENE TECH
Real-time fluorescence quantitative PCR detection kit for quantitative detection of GGPPS1 genes, detection method and application
InactiveCN103937886AImplement early screeningImplement early treatmentMicrobiological testing/measurementLiver tissueDiagnosis standards
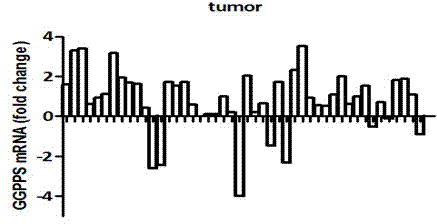
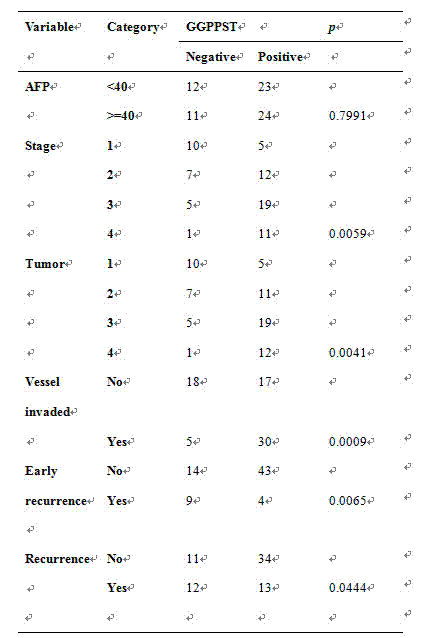
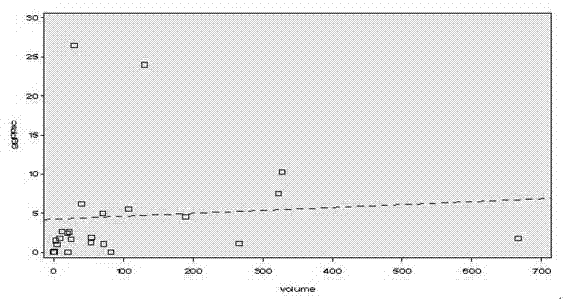
The invention discloses a fluorescence quantitative PCR kit for detecting GGPPS1 in a liver tissue of a liver cancer sample. The kit comprises the following ingredients: a specific primer and a fluorescence quantitative PCR reaction solution. The invention further discloses a using method of the fluorescence quantitative PCR kit for detecting the GGPPS1 in the liver tissue. By utilizing the kit, the expression of the GGPPS1 in the liver tissue can be fast and quantitatively detected, and the detection result can be used as a diagnosis standard of clinical liver cancer in early stage or advanced stage. The kit disclosed by the invention has the advantages of simplicity and convenience in operation, high speed, stable result, high sensitivity and strong specificity.
Owner:NANJING UNIV
Messenger ribonucleic acid (mRNA) level in-situ hybridization detection kit for JAGGED1 in early stage of pathological change of breast cancer bone metastasis, and detection method and application
InactiveCN102559887AHigh sensitivityStrong specificityMicrobiological testing/measurementIn situ hybridizationRNA
The invention discloses an in-situ hybridization detection kit which comprises a hybridization probe and a marker, and a method for performing in-situ hybridization detection on messenger ribonucleic acid (mRNA) of signal transducer protein (Jagged1), which is closely related with the pathologic evolution of the early stage of breast cancer bone metastasis by using the kit. The method comprises the following steps of: (1) contacting RNA to be detected in substrate and the hybridization probe under the condition that the hybridization probe and a target sequence can form a stable hybridization complex to form the hybridization complex; and (2) detecting the hybridization complex. By the kit and the detection method, the expression level of JAGGED1 gene can be detected at the mRNA level; a detection index is earlier than the detection indexes of medical imaging and the conventional clinical chemistry; real mRNA-level screening of the early stage of canceration can be realized; and meanwhile, the detection method is simple and convenient, and low in cost and can be conveniently popularized and applied in county hospitals.
Owner:NATUREGEN BIOTECH SHANGHAI
In-situ hybridization testing kit of ALDH1 gene and testing method and application thereof
InactiveCN101993944AHigh sensitivityStrong specificityMicrobiological testing/measurementIn situ hybridizationAldehyde dehydrogenase
The invention relates to an in-situ hybridization testing kit of an aldehyde dehydrogenase (ALDH1) gene. The kit comprises a hybridization probe and a marker, wherein the sequence of the hybridization probe is shown as SEQ ID No. 1. The invention also provides an in-situ hybridization testing method of the ALDH1gene. In addition, the invention also provides application of the kit to preparation of medicaments for testing breast cancer or prognostic diseases of breast cancer. The kit provided by the invention has the characteristics of high flexibility and specificity. The testing method of the invention is convenient and easy to operate and can be generally used and popularized in district-level or city-level hospitals.
Owner:SUZHOU FUYING GENE TECH
In-situ hybridization detection kit and detection method of kallikrein-related peptidase 2 (KLK2) gene and application of detection kit
InactiveCN101988109AHigh sensitivityStrong specificityMicrobiological testing/measurementDiseaseHybridization probe
The invention relates to an in-situ hybridization detection kit of a kallikrein-related peptidase 2 (KLK2 / hK2) gene. The detection kit comprises a hybridization probe and a marker, wherein the sequence of the hybridization probe is shown as SEQ ID NO.1. The invention also provides an in-situ hybridization detection method of the KLK2 gene. Moreover, the invention also provides the application of the kit to the preparation of a medicament for detecting prostatic cancer. The invention has the advantages that: the kit provided by the invention has the characteristics of high sensitivity and high specificity; and the detection method of the invention is convenient and easy to operate and can be commonly used and popularized in hospitals of district level or above.
Owner:SUZHOU FUYING GENE TECH
Kit for assaying MICRORNA-34 level in early stage of pathologic evolution of various cancers through in situ hybridization and assay method and application
InactiveCN102443643AHigh sensitivityStrong specificityMicrobiological testing/measurementIn situ hybridisationAssay
The invention discloses an in situ hybridization assay kit. The kit comprises a hybridization probe and a label. The invention also discloses a method for assaying microRNA-34 closely related to early-stage pathologic evolution of various cancers through in situ hybridization by using the kit. The method comprises the following steps: (1) under the condition that the hybridization probe and a target sequence can form a stable hybrid complex, contacting the RNA to be assayed in a substrate with the hybridization probe to form the hybrid complex; and (2) assaying the hybrid complex. The kit and assay method disclosed by the invention have the following beneficial effects: the microRNA-34 expression can be assayed on the RNA level and is earlier than the medical imaging and existing clinical biochemical assay indexes, so that real RNA level screening in the early stage of cancerization can be realized; and meanwhile, the assay method is simple and convenient, has low cost and is convenient to popularize and apply in the county and district level hospitals.
Owner:SUZHOU FUYING GENE TECH
Cancer pathologic evolution early-stage Gankyrin gene mRNA level in-situ hybridization detection kit, detection method and applications thereof
InactiveCN104711331AImplement early screeningImplement early treatmentMicrobiological testing/measurementHybridization probeMedical imaging technology
The invention discloses an in-situ hybridization detection kit, which comprises a hybridization probe and a marker, and further discloses a method utilizing the in-situ hybridization detection kit to detect the Gankyrin gene RNA expression change, which is closely related with the pathologic evolution in the early stage of cancer. The method comprises the following steps: (1) under a condition that a hybridization probe and a target sequence can form a stable hybridization complex, contacting RNA to be detected in a substrate with a hybridization probe so as to form a hybridization complex; (2) detecting the obtained hybridization complex. The provided kit and detection method can detect the expression quantity of Gankyrin gene in the RNA level, can detect the cancer in an earlier stage compared with the medical imaging technology and conventional clinical biochemical index detection, can achieve real RNA level screening in the early stage of cancer, moreover, have the advantages of simpleness, convenience, and low cost, and thus are convenient to promote and use in county / district hospitals.
Owner:NATUREGEN BIOTECH SHANGHAI
In-situ hybridization detection kit of miR-378 in early stage of pathologic evolution of gastric cancer, detection method and application
InactiveCN104131064AHigh sensitivityStrong specificityMicrobiological testing/measurementHybridization probeBiology
The invention discloses an in-situ hybridization detection kit which includes a hybridization probe and a marker. The invention also discloses a method for detecting change of expression of a miR-378 gene, which is closely relative to pathologic evolution in an early stage of gastric cancer cancerization, through the kit in a manner of in-situ hybridization detection. The method includes following steps: (1) on the condition that the hybridization probe and a target sequence can form a stable hybridization complex, contacting a to-be-detected miR-378 in a substrate with the hybridization probe to form the hybridization complex; and (2) detecting the hybridization complex. By means of the kit and the method, an expression quantity of the miR-378 can be detected on a miRNA level. Compared with image medicine and a clinical biochemical detection index in the prior art, The kit and the method can be applied in an earlier stage and can achieve a true RNA-level screening operation during the early stage of cancerization. Meanwhile, the detection method is simple and convenient, is low in cost and is suitable for being popularized in hospitals in counties or districts.
Owner:嘉兴瑞康生物科技有限公司
Maspin gene in-situ hybridization detection kit and detection method and use thereof
InactiveCN101993937AHigh sensitivityStrong specificityMicrobiological testing/measurementHybridization probeMaspin Gene
The invention relates to a Maspin gene in-situ hybridization detection kit, which comprises a hybridization probe and markers, wherein the sequence of the hybridization probe is represented by the sequence SEQ ID No.1. The invention also provides a Maspin gene in-situ hybridization detection method and the use of the kit in the preparation of medicaments for detecting breast cancer. The invention has the advantage that the kit provided by the invention has the characteristics of high sensitivity and high specificity. The detection method of the invention is convenient and simple for operation and can be widely used and promoted in district or above hospitals.
Owner:SUZHOU FUYING GENE TECH
In situ hybridization detection kit for HER-2 gene, detection method and application thereof
InactiveCN101993938AHigh sensitivityStrong specificityMicrobiological testing/measurementHybridization probeMicrobiology
The invention relates to an in situ hybridization detection kit for a human epidermal growth factor receptor-2 (HER-2) gene. The kit comprises a hybridization probe and a marker, wherein the sequence of the hybridization probe is shown as SEQ ID NO.1. The invention also provides an in situ hybridization detection method for the Heparinase gene. In addition, the invention also provides the application of the kit in preparing medicaments for detecting diseases such as breast cancer. The in situ hybridization detection kit for the HER-2 gene and the detection method have the advantages that: the kit provided by the invention has the characteristics of high sensitivity and high specificity; and the detection method of the invention is convenient and easy to operate, and can be commonly used and popularized in district-level above hospitals.
Owner:SUZHOU FUYING GENE TECH
Solid tumor pathologic evolution early-stage GADD45G gene mRNA level in-situ hybridization detection kit, detection method and applications thereof
InactiveCN104711336AHigh sensitivityStrong specificityMicrobiological testing/measurementHybridization probeMedical imaging technology
The invention discloses an in-situ hybridization detection kit, which comprises a hybridization probe and a marker, and further discloses a method utilizing the in-situ hybridization detection kit to detect the GADD45G (growth arrest and DNA-damage-inducible, gamma) gene RNA expression change, which is closely related with the pathologic evolution in the early stage of solid tumor. The method comprises the following steps: (1) under a condition that a hybridization probe and a target sequence can form a stable hybridization complex, contacting RNA to be detected in a substrate with a hybridization probe so as to form a hybridization complex; (2) detecting the obtained hybridization complex. The provided kit and detection method can detect the expression quantity of GADD45G gene in the RNA level, can detect the cancer in an earlier stage compared with the medical imaging technology and conventional clinical biochemical index detection, can achieve real RNA level screening in the early stage of canceration, moreover, have the advantages of simpleness, convenience, and low cost, and thus are convenient to promote and use in county / district hospitals.
Owner:NATUREGEN BIOTECH SHANGHAI
In-situ hybridization detection kit for MICRORNA (MICRO Ribonucleic Acid)-145 level at pathologic evolution early stages of various colon cancers as well as microRNA-145 in-situ hybridization detection method and application of microRNA-145 to preparation of in-situ hybridization detection kit for colon cancers
InactiveCN102443647AImplement early screeningImplement early treatmentMicrobiological testing/measurementIn situ hybridisationHybridization probe
The invention discloses an in-situ hybridization detection kit, which comprises a hybridization probe and a marker. The invention also discloses a method for detecting microRNA (micro Ribonucleic Acid)-145 which is closely correlated with the pathologic evolution at an early stage of colon cancer by using in-situ hybridization through the in-situ hybridization detection kit. The method comprises the following steps of: firstly, under the condition that the hybridization probe and a target sequence can form a stable hybrid complex, contacting RNA to be detected in a substrate with the hybridization probe to form a hybrid complex; and secondly, detecting the hybrid complex. According to the in-situ hybridization detection kit and the detection method disclosed by the invention, the expression of microRNA-145 can be detected in the RNA level, which is earlier than imaging medicine and traditional clinical biochemical detection index and the real RNA level screening at the early stage of canceration can be realized; and meanwhile, the detection method disclosed by the invention is simple and convenient, is low in cost and is convenience for popularization and application to county and district level hospitals.
Owner:SUZHOU FUYING GENE TECH
In situ hybridization detection kit for early uterine cancer
ActiveCN101469352BHigh sensitivityStrong specificityMicrobiological testing/measurementHybridization probeMicrobiology
The invention relates to an in situ hybridization detection kit for early uterine cancer, comprising a hybridization probe and a marker, and the sequence of the hybridization probe is shown in SEQ ID NO.1. The invention also provides an in situ hybridization detection method of the P2RX7 gene. In addition, the present invention also provides the application of the kit in the preparation of drugs for detecting uterine cancer. The invention has the advantages that: the kit provided by the invention has the characteristics of high sensitivity and strong specificity. The detection method of the invention is convenient and simple to operate, and can be widely used and popularized in hospitals above the district level.
Owner:台州和和生物科技有限公司
In-situ hybridization assay kit for mRNA level of premalignant CD151 as well as assay method and application
InactiveCN102605057AHigh sensitivityStrong specificityMicrobiological testing/measurementHybridization probeTetraspanin
The invention discloses an in-situ hybridization assay kit, comprising a hybridization probe and a marker. The invention also discloses a method for assaying mRNA of a tetraspanin (CD151) gene closely related with the premalignant pathology evolution through in-situ hybridization using the kit, and the method comprises the following steps: (1) contacting RNA to be assayed in a substrate with the hybridization probe to form a hybridization complex under a condition in which the hybridization probe and a target sequence can form a stable hybridization complex; and (2) assaying the hybridization complex. By using the kit and the assay method of the invention, the expression level of the CD151 gene can be assayed in the mRNA level, the assay is earlier than medical imaging and traditional clinical biochemical detection indexes, and screening the premalignant mRNA level is really realized. In addition, the assay method of the invention is simple and convenient, is low in cost, and is convenient to be popularized and applied in county and district hospitals.
Owner:NATUREGEN BIOTECH SHANGHAI
Cancer pathologic evolution early-stage GPR116 gene mRNA level in-situ hybridization detection kit, detection method and applications thereof
InactiveCN104711327AHigh sensitivityStrong specificityMicrobiological testing/measurementHybridization probeDistrict hospital
The invention discloses an in-situ hybridization detection kit, which comprises a hybridization probe and a marker, and further discloses a method utilizing the in-situ hybridization detection kit to detect the GPR116 gene RNA expression change, which is closely related with the pathologic evolution in the early stage of cancer. The method comprises the following steps: (1) under a condition that a hybridization probe and a target sequence can form a stable hybridization complex, contacting RNA to be detected in a substrate with a hybridization probe so as to form a hybridization complex; (2) detecting the obtained hybridization complex. The provided kit and detection method can detect the expression quantity of GPR116 gene in the RNA level, can detect the cancer in an earlier stage compared with the medical imaging technology and conventional clinical biochemical index detection, can achieve real RNA level screening in the early stage of cancer, moreover, have the advantages of simpleness, convenience, and low cost, and thus are convenient to promote and use in county / district hospitals.
Owner:NATUREGEN BIOTECH SHANGHAI
Cancer metastasis pathologic evolution early-stage HOPX gene mRNA level in-situ hybridization detection kit, detection method and applications thereof
InactiveCN104711335AHigh sensitivityStrong specificityMicrobiological testing/measurementHybridization probeDistrict hospital
The invention discloses an in-situ hybridization detection kit, which comprises a hybridization probe and a marker, and further discloses a method utilizing the in-situ hybridization detection kit to detect the HOPX gene RNA expression change, which is closely related with the pathologic evolution in the early stage of cancer metastasis. The method comprises the following steps: (1) under a condition that a hybridization probe and a target sequence can form a stable hybridization complex, contacting RNA to be detected in a substrate with a hybridization probe so as to form a hybridization complex; (2) detecting the obtained hybridization complex. The provided kit and detection method can detect the expression quantity of HOPX gene in the RNA level, can detect the cancer metastasis in an earlier stage compared with the medical imaging technology and conventional clinical biochemical index detection, can achieve real RNA level screening in the early stage of cancer metastasis, moreover, have the advantages of simpleness, convenience, and low cost, and thus are convenient to promote and use in county / district hospitals.
Owner:NATUREGEN BIOTECH SHANGHAI
HELQ gene mRNA level in-situ hybridization detection kit, detection method and applications thereof
InactiveCN104711337AImplement early screeningImplement early preventionMicrobiological testing/measurementHybridization probeDistrict hospital
The invention discloses an in-situ hybridization detection kit, which comprises a hybridization probe and a marker, and further discloses a method utilizing the in-situ hybridization detection kit to detect the HELQ gene RNA expression change, which is closely related with the pathologic evolution in the early stage of ovarian cancer. The method comprises the following steps: (1) under a condition that a hybridization probe and a target sequence can form a stable hybridization complex, contacting RNA to be detected in a substrate with a hybridization probe so as to form a hybridization complex; (2) detecting the obtained hybridization complex. The provided kit and detection method can detect the expression quantity of HELQ gene in the RNA level, can detect the cancer in an earlier stage compared with the medical imaging technology and conventional clinical biochemical index detection, can achieve real RNA level screening in the early stage of ovarian cancer, moreover, have the advantages of simpleness, convenience, and low cost, and thus are convenient to promote and use in county / district hospitals.
Owner:NATUREGEN BIOTECH SHANGHAI
In-situ hybridization detection kit of MAGEA3 (Melanoma-Associated Antigen Antiger 3) as well as detection method and application thereof
InactiveCN101988096AHigh sensitivityStrong specificityMicrobiological testing/measurementDiseaseAntigen
The invention relates to an in-situ hybridization detection kit of MAGEA3 (Melanoma-Associated Antigen Antiger 3), comprising a hybridization probe and a marker, wherein the hybridization probe sequence is shown in SEQ ID No.1. The invention also provides an in-situ hybridization detection method of the MAGEA3. In addition, the invention also provides application of the kit to the preparation of medicaments for detecting liver cancer diseases. The kit provided by the invention has the advantages of high sensitivity and strong specificity; and the detection method of the invention is convenient and simple for operation and can be widely used and popularized in municipal or higher-grade hospitals.
Owner:NATUREGEN BIOTECH SHANGHAI
In-situ hybridization detection kit for microRNA-182 level in early stage of pathological evolution of various cancers, and detection method and application thereof
InactiveCN102443648AHigh sensitivityStrong specificityMicrobiological testing/measurementHybridization probeMicroRNA
The invention discloses an in-situ hybridization detection kit which comprises a hybridization probe and markers. The invention also discloses a method for applying the kit in in-situ hybridization detection of micro RNA-182 having a close relationship with pathological evolution of various cancers in an early stage, and the method comprises the following steps: (1) under the condition that the hybridization probe and a target sequence can form a stable hybrid complex, contacting RNA to be detected in a substrate with the hybridization probe so as to form a hybrid complex; (2) detecting the hybrid complex. The kit and the detection method provided in the invention can detect the expression level of micro RNA-182 at RNA level, enable detection indexes to be obtained at an earlier stage than medical imaging and conventional clinical biochemical detection do, and can realize genuine precancerous lesion RNA level screening; meanwhile, the detection method is simple and convenient, costs little and is convenient for popularization and application in county hospitals.
Owner:SUZHOU FUYING GENE TECH
HDAC10 gene in-situ hybridization detection kit, detection method and application thereof
InactiveCN104711322AHigh sensitivityStrong specificityMicrobiological testing/measurementHybridization probeHistone deacetylase
The invention relates to a HDAC10 (Histone Deacetylase 10) gene mRNA in-situ hybridization detection kit, which comprises a hybridization probe and a marker; wherein the sequence of the hybridization probe is represented by the SEQ ID No. AF426160. The invention further provides a HDAC10 gene in-situ hybridization detection method. Besides, the invention also provides an application of the kit in preparation of drugs for treating cervical cancer. The provided kit has the characteristics of high sensitivity and strong specificity; and the detection method has the advantages of simple and convenient operation and can be commonly used and promoted in district hospitals and better hospitals.
Owner:NATUREGEN BIOTECH SHANGHAI
In-situ hybridization detection kit of DAXX gene as well as detection method and application thereof
InactiveCN101988090AHigh sensitivityStrong specificityMicrobiological testing/measurementHybridization probeMicrobiology
The invention relates to an in-situ hybridization detection kit of a DAXX (death associated protein) gene, which comprises a hybridization probe and a marker, wherein the sequence of the hybridization probe is as shown in SEQ ID NO.1. The invention also discloses an in-situ hybridization detection of the DAXX gene. In addition, the invention also provides an application of the kit in preparing drugs for detecting acute lymphoblastic leukemia. The invention has the advantage that the kit has the characteristics of high sensitivity and strong specificity. The detection method in the invention is convenient and simple in operation, and can be widely applied and popularized in district-level or city-level hospitals.
Owner:NATUREGEN BIOTECH SHANGHAI
AGPS gene mRNA level in-situ hybridization detection kit, detection method and applications thereof
InactiveCN104711325AHigh sensitivityStrong specificityMicrobiological testing/measurementIn situ hybridizationCarcinogenesis
The invention discloses an in-situ hybridization detection kit, which comprises a hybridization probe and a marker, and further discloses a method utilizing the in-situ hybridization detection kit to detect the AGPS (alkylglycerone phosphate synthase, AGPS) gene RNA expression change, which is closely related with the pathologic evolution in the early stage of canceration. The method comprises the following steps: (1) under a condition that a hybridization probe and a target sequence can form a stable hybridization complex, contacting RNA to be detected in a substrate with a hybridization probe so as to form a hybridization complex; (2) detecting the obtained hybridization complex. The provided kit and detection method can detect the expression quantity of AGPS gene in the RNA level, can detect the cancer in an earlier stage compared with the medical imaging technology and conventional clinical biochemical index detection, can achieve real RNA level screening in the early stage of canceration, moreover, have the advantages of simpleness, convenience, and low cost, and thus are convenient to promote and use in county / district hospitals.
Owner:NATUREGEN BIOTECH SHANGHAI
In-situ hybridization detection kit for MICRORNA (MICRO Ribonucleic Acid)-233 level at pathologic evolution early stage of colon cancer as well as microRNA-233 in-situ hybridization detection method and application of microRNA-233 to preparation of in-situ hybridization detection kit for colon cancer
InactiveCN102443650AHigh sensitivityStrong specificityMicrobiological testing/measurementIn situ hybridizationCarcinogenesis
The invention discloses an in-situ hybridization detection kit, which comprises a hybridization probe and a marker. The invention also discloses a method for detecting microRNA (micro Ribonucleic Acid)-233 which is closely correlated with the pathologic evolution at an early stage of colon cancer by using in-situ hybridization through the in-situ hybridization detection kit. The method comprises the following steps of: firstly, under the condition that the hybridization probe and a target sequence can form a stable hybrid complex, contacting RNA to be detected in a substrate with the hybridization probe to form a hybrid complex; and secondly, detecting the hybrid complex. According to the in-situ hybridization detection kit and the detection method disclosed by the invention, the expression of microRNA-233 can be detected in the RNA level, which is earlier than imaging medicine and traditional clinical biochemical detection index and the real RNA level screening at the early stage of canceration can be realized; and meanwhile, the detection method disclosed by the invention is simple and convenient, is low in cost and is convenience for popularization and application to county and district level hospitals.
Owner:SUZHOU FUYING GENE TECH
Horizontal in-situ hybridization detection kit and detection method as well as application for MICRORNA17-3P in earlier stage of cancer pathologic evolution
InactiveCN102533984AImplement early screeningImplement early treatmentMicrobiological testing/measurementHybridization probeClinical biochemistry
The invention discloses an in-situ hybridization detection kit which comprises a hybridization probe and a marker. The invention also discloses an in-situ hybridization detection method for microRNA-17-3p being closely related to the early pathologic evolution of various cancers by using the kit. The in-situ hybridization detection method comprises the following steps of: (1) under the condition that the hybridization probe and a target sequence can form a stable hybridization complex, leading RNA (Ribonucleic Acid) to be tested in a substrate to be in contact with the hybridization probe, and forming the hybridization complex; and (2) detecting the hybridization complex. The kit and the detection method provided by the invention can be used for detecting the expression quantity of the microRNA17-3p at the level of RNA, which is more early obtained in comparison with detection indexes of imaging medicine and the traditional clinical biochemistry, thereby the RNA level screening can be realized truly in the earlier stage of canceration, and simultaneously, the detection method provided by the invention is simple and convenient and low in cost and is convenient to popularize and apply in county-level hospitals.
Owner:SUZHOU FUYING GENE TECH
Multi-cancer pathologic evolution early-stage Bmi1 gene mRNA level in-situ hybridization detection kit, detection method and applications thereof
InactiveCN104711326AEarly preventionImplement early screeningMicrobiological testing/measurementHybridization probeDistrict hospital
The invention discloses an in-situ hybridization detection kit, which comprises a hybridization probe and a marker, and further discloses a method utilizing the in-situ hybridization detection kit to detect the Bmi1 gene RNA expression change, which is closely related with the pathologic evolution in the early stage of multiple cancers. The method comprises the following steps: (1) under a condition that a hybridization probe and a target sequence can form a stable hybridization complex, contacting RNA to be detected in a substrate with a hybridization probe so as to form a hybridization complex; (2) detecting the obtained hybridization complex. The provided kit and detection method can detect the expression quantity of Bmi1 gene in the RNA level, can detect the cancers in an earlier stage compared with the medical imaging technology and conventional clinical biochemical index detection, can achieve real RNA level screening in the early stage of cancers, moreover, have the advantages of simpleness, convenience, and low cost, and thus are convenient to promote and use in county / district hospitals.
Owner:NATUREGEN BIOTECH SHANGHAI
Horizontal in-situ hybridization detection kit and detection method as well as application for MICRORNA-143 in earlier stage of cancer pathologic evolution
InactiveCN102533987AHigh sensitivityStrong specificityMicrobiological testing/measurementHybridization probeClinical biochemistry
The invention discloses an in-situ hybridization detection kit which comprises a hybridization probe and a marker. The invention also discloses an in-situ hybridization detection method for microRNA-143 being closely related to the early pathologic evolution of various cancers by using the kit. The in-situ hybridization detection method comprises the following steps of: (1) under the condition that the hybridization probe and a target sequence can form a stable hybridization complex, leading RNA (Ribonucleic Acid) to be tested in a substrate to be in contact with the hybridization probe, and forming the hybridization complex; and (2) detecting the hybridization complex. The kit and the detection method provided by the invention can be used for detecting the expression quantity of the microRNA17-143 at the level of RNA, which is more early obtained in comparison with detection indexes of imaging medicine and the traditional clinical biochemistry, thereby the RNA level screening can be realized truly in the earlier stage of canceration, and simultaneously, the detection method provided by the invention is simple and convenient and low in cost and is convenient to popularize and apply in county-level hospitals.
Owner:SUZHOU FUYING GENE TECH
MRNA level in-situ hybridization detection kit of KLF4 gene in earlier stage of pathologic evolution of human cardiovascular and cerebtovascular disease, detection method and application
InactiveCN104141000AImplement early screeningImplement early preventionMicrobiological testing/measurementDiseaseHybridization probe
The invention discloses an in-situ hybridization detection kit which comprises a hybridization probe and a marker. The invention also discloses a method for in-situ hybridization detection of vascular endothelial cell's genetic factor (KLF4) gene's mRNA which is closely related with pathologic evolution of cardiovascular and cerebtovascular vascular endothelial injury in the earlier stage by the use of the kit. The method comprises the following steps: (1) under the condition that a hybridization probe and a target sequence can form a stable hybrid complex, RNA to be tested in a substrate is contacted with the hybridization probe so as to form a hybrid complex; and (2) the hybrid complex is detected. According to the detection kit and the detection method, gene expression quantity can be detected at the RNA level, the index of the detection method is ealier than present clinical biochemical detection index (protein detection index), and genuine RNA level screening of the earlier stage of cardiovascular and cerebtovascular disease can be realized. Meanwhile, the detection method provided by the invention is simple and convenient; cost is low; and the method is convenient for popularization and application in district-level hospitals.
Owner:嘉兴瑞康生物科技有限公司
In-situ hybridization detection kit for MDM2 genes and detection method and application thereof
InactiveCN101993936AHigh sensitivityStrong specificityMicrobiological testing/measurementHybridization probeMicrobiology
The invention relates to an in-situ hybridization detection kit for MDM2 genes, which comprises a hybridization probe and a marker, wherein the sequence of the hybridization probe is expressed as SEQ ID No.1. The invention also provides an in-situ hybridization detection method for the MDM2 genes. In addition, the invention also provides application of the kit in preparation of a medicament for detecting breast cancer diseases. The kit provided by the invention has the advantages of high sensitivity and strong specificity. The detection method is convenient and easy to operate, and can be universally used and popularized in hospitals of above district level.
Owner:NATUREGEN BIOTECH SHANGHAI
Popular searches
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com