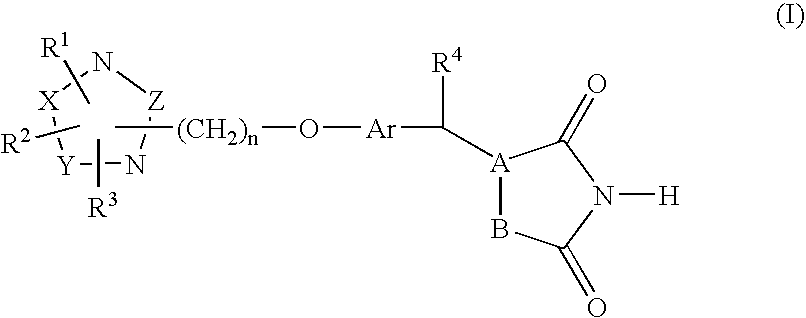
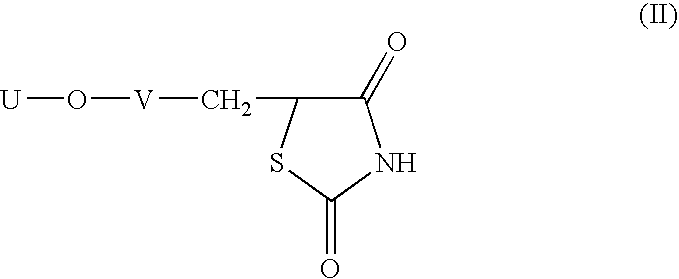
Heterocyclic compounds, process for their preparation and pharmaceutical compositions containing them and their use in the treatment of diabetes and related diseases
a technology of heterocyclic compounds and compounds, applied in the field of heterocyclic compounds, can solve the problems of increasing restrictions, limiting the use of antidiabetic treatment, and raising doubts about the intrinsic suitability of antidiabetic treatment, so as to reduce the toxic effect, no toxic effect, and enhance the effect of activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
preparation 1
4-[2-[4-Methyl-2-propyl-6-oxo-1,6-dihydro-1-pyrimidinyl]ethoxy]benzaldehyde
[0156]
[0157]To a stirred suspension of NaH (570 mg, 22.57 mmol, 95%) in dry DMF (35 ml) at 25° C. was added a solution of 4-methyl-2-propyl-1,6-dihydro-6-pyrimidione (2.64 g, 17.36 mmol) in dry DMF. After the effervescence has ceased, anhydrous LiBr (3.51 g, 40.0 mmol) was added followed by 4-[2-bromoethoxy]benzaldehyde (4.37 g, 19.08 mmol) in dry DMF at the same temperature. The reaction mixture was immersed in a preheated oil bath at 70° C. and stirred for 2 h. The reaction mixture was cooled to room temperature, poured into water and extracted with EtOAc. The combined EtOAc layers were washed with brine, dried over anhydrous Na2SO4 and concentrated. The crude compound was chromatographed over silica gel using 3:7 EtOAc-pet. ether as eluent to obtain the title compound (1.61 g, 31%).
[0158]1H NMR (CDCl3): δ 9.80 (s, 1H), 7.82 (d, J=8.72 Hz, 2H), 6.95 (d, J=8.72 Hz, 2H), 6.20 (s, 1H), 4.45 (t, J=5.30 Hz, 2H),...
preparation 2
4-[2-[2,4-Dimethyl-6-oxo-1,6-dihydro-1-pyrimidinyl]ethoxy]benzaldehyde
[0159]
[0160]The title compound (0.8 g, 30%) was prepared from 2,4-dimethyl-1,6-dihydro-6-pyrimidione (1,3 g, 10.48 mmol) and 4-[2-bromoethoxy]benzaldehyde (2.4 g, 10.48 mmol) in the presence of a base K2 CO3 (2.89 g, 20.96 mmol) by a similar procedure as described in preparation 1.
[0161]1H NMR (CDCl3): δ 9.90 (s, 1H), 7.80 (d, J=8.70 Hz, 2H), 7.02 (d, J=8.70 Hz, 2H), 6.20 (s, 1H), 4.50-4.30 (m, 4H), 2.70 (s,3H), 2.20 (s, 3H).
preparation 3
4-[2-[2-Ethyl-4-methyl-6-oxo-1,6-dihydro-1-pyrimidinyl]ethoxy]benzaldehyde
[0162]
[0163]The title compound (1.7 g, 42%) was prepared from 2-ethyl-4-methyl-1,6-dihydro-6-pyrimidone (2.0 g, 14.49 mmol), 4-[2-bromoethoxy]benzaldehyde (332 g, 14.19 mmol). LIBr (2.9 g, 33.33 mmol) and NaH (0.45 g, 18.84 mmol) as base, by a similar procedure to that described in preparation 1.
[0164]1H NMR (CDCl3): δ 9.90 (s, 1H), 7.80 (d, J=8.70 Hz, 2H), 6.98 (d, J=8.70 Hz, 2H), 6.20 (s, 1H), 4.52-4.25 (m, 4H), 3.02 (q, J=7.40 Hz, 2H), 2.30 (s, 3H), 1.40 (t, J=7.40 Hz, 3H).
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com