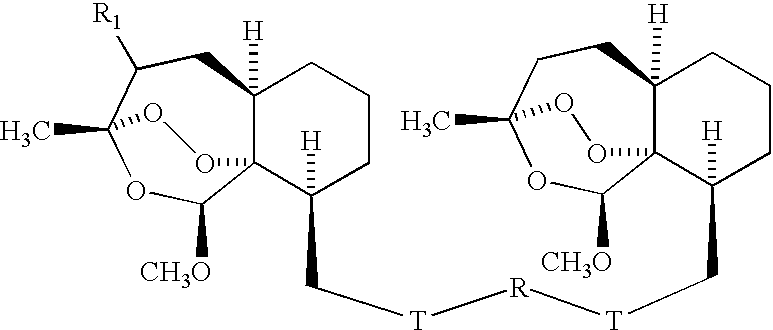
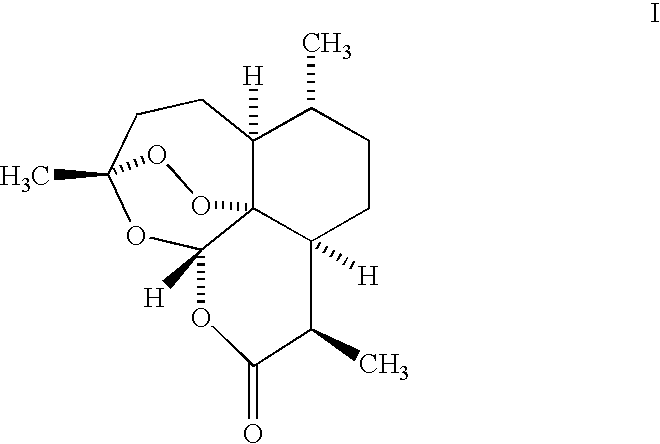
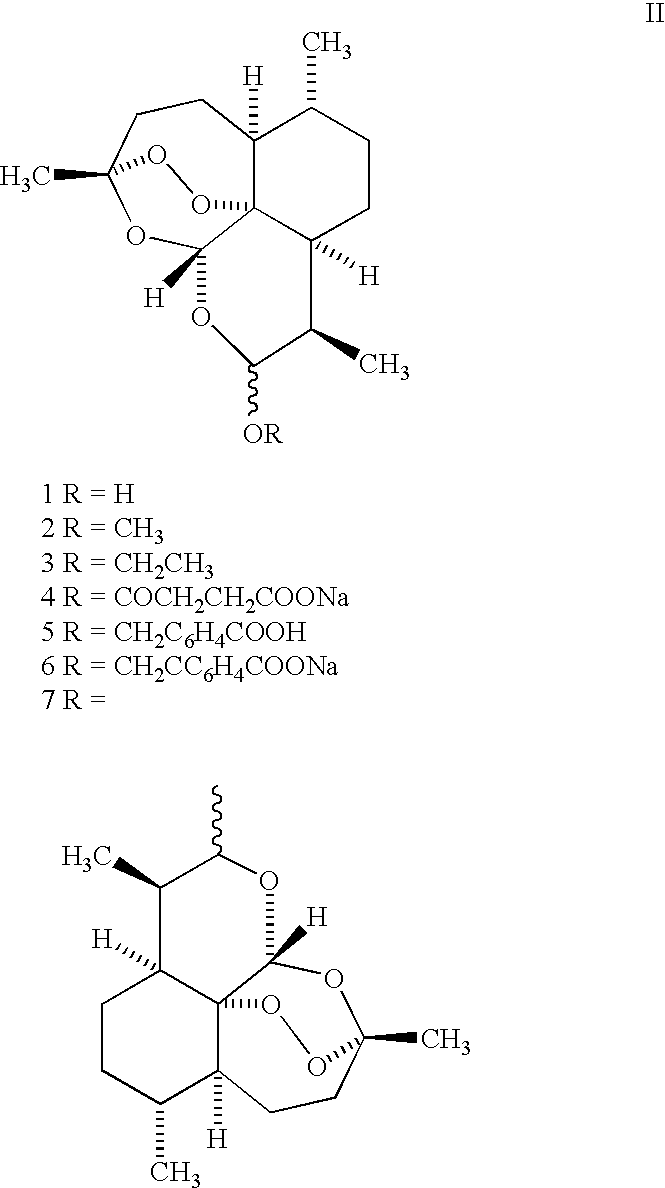
Trioxane dimer compound having antiproliferative and antitumor activities
a trioxane dimer and antiproliferative technology, applied in the field of new trioxane dimers, can solve the problems of difficult task for scientists to modify qhs chemically
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of Trioxane Ether Dimer (6)
A flame-dried 10 mL round-bottomed flash was charged with 2,6-di-tert-butyl-4-methylpyridine (30.0 mg, 0.15 mmole) and methylene chloride (2 mL) at room temperature. To this mixture at 0.degree. C. was added trifluoromethanesulfonic anhydride (25.0 .mu.L, 0.15 mmole) via gas-tight syringe. A solution of trioxane alcohol (20.0 mg, 0.07 mmole) in methylene chloride (0.5 mL) was cooled to 0.degree. C. and added to the reaction mixture via cannula. The reaction was stirred at 0.degree. C. for 3 hours. The reaction was monitored by TLC until all trioxane alcohol was consumed.
The reaction was quenched with saturated sodium bicarbonate (3 mL) at 0.degree. C. and diluted with methylene chloride (5 mL). Two layers were separated and the aqueous phase was extracted with methylene chloride (3.times.5 mL). The combined organic layers were washed with brine (15 mL), dried over magnesium sulfate, filtered and concentrated under reduced pressure. The crude pr...
example 2
Preparation of Trioxane Dimer (7)
To a flame-dried round-bottomed flask charged with 2,6-di-t-butyl-4-methylpyridine (22 mg, 0.11 mmol) in dry methylene chloride (1 mL) at 0.degree. C. was added freshly opened triflic anhydride (18 .mu.L, 0.11 mmol) via a syringe under argon atmosphere. After being stirred for 5 minutes the reaction mixture was slowly treated with a precooled solution of 4.beta.-methyltrioxane alcohol (15 mg, 0.053 mmol) in methylene chloride (0.5 mL) at 0.degree. C. via a cannula. The resultant mixture was stirred for 2.5 hours at 0.degree. C., quenched with water (3 mL) at 0.degree. C. and diluted with ether (5 mL), the organic layer was separated, and the aqueous layer was extracted twice with ether (5 mL.times.2). The combined organic layer was washed with brine solution (5 mL), and dried over anhydrous magnesium sulfate, filtered, and concentrated under reduced pressure to yield a crude product which was purified by silica gel column chromatography using 2:98 et...
example 3
Preparation of Trioxane Dimer (8)
To a flame-dried round-bottomed flask charged with 2,6-di-t-butyl-4-methylpyridine (22 mg, 0.11 mmol) in dry methylene chloride (1 mL) at 0.degree. C. was added freshly opened triflic anhydride (18 .mu.L, 0.11 mmol) via a syringe under argon atmosphere. After being stirred for 5 minutes the reaction mixture was slowly treated with a precooled solution of 4.beta.-benzyltrioxane alcohol (15 mg, 0.053 mmol) in methylene chloride (0.5 mL) at 0.degree. C. via a cannula. The resultant mixture was stirred for 2.5 hours at 0.degree. C., quenched with water (3 mL) at 0.degree. C. and diluted with ether (5 mL), the organic layer was separated, and the aqueous layer was extracted twice with ether (5 mL.times.2). The combined organic layer was washed with brine solution (5 mL), and dried over anhydrous magnesium sulfate, filtered, and concentrated under reduced pressure to yield a crude product which was purified by silica gel column chromatography using 2:98 et...
PUM
| Property | Measurement | Unit |
|---|---|---|
| time | aaaaa | aaaaa |
| temperatures | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com