Process for improved water flux through a TFC membrane
a technology of thin film composite and water flux, applied in the field of thin film composite (tfc) membranes, can solve problems such as blistered membranes that were susceptible to breakage, and achieve the effects of improving water flux, improving water flux performance, and preserving tfc membrane performance properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of Sea Water Membrane
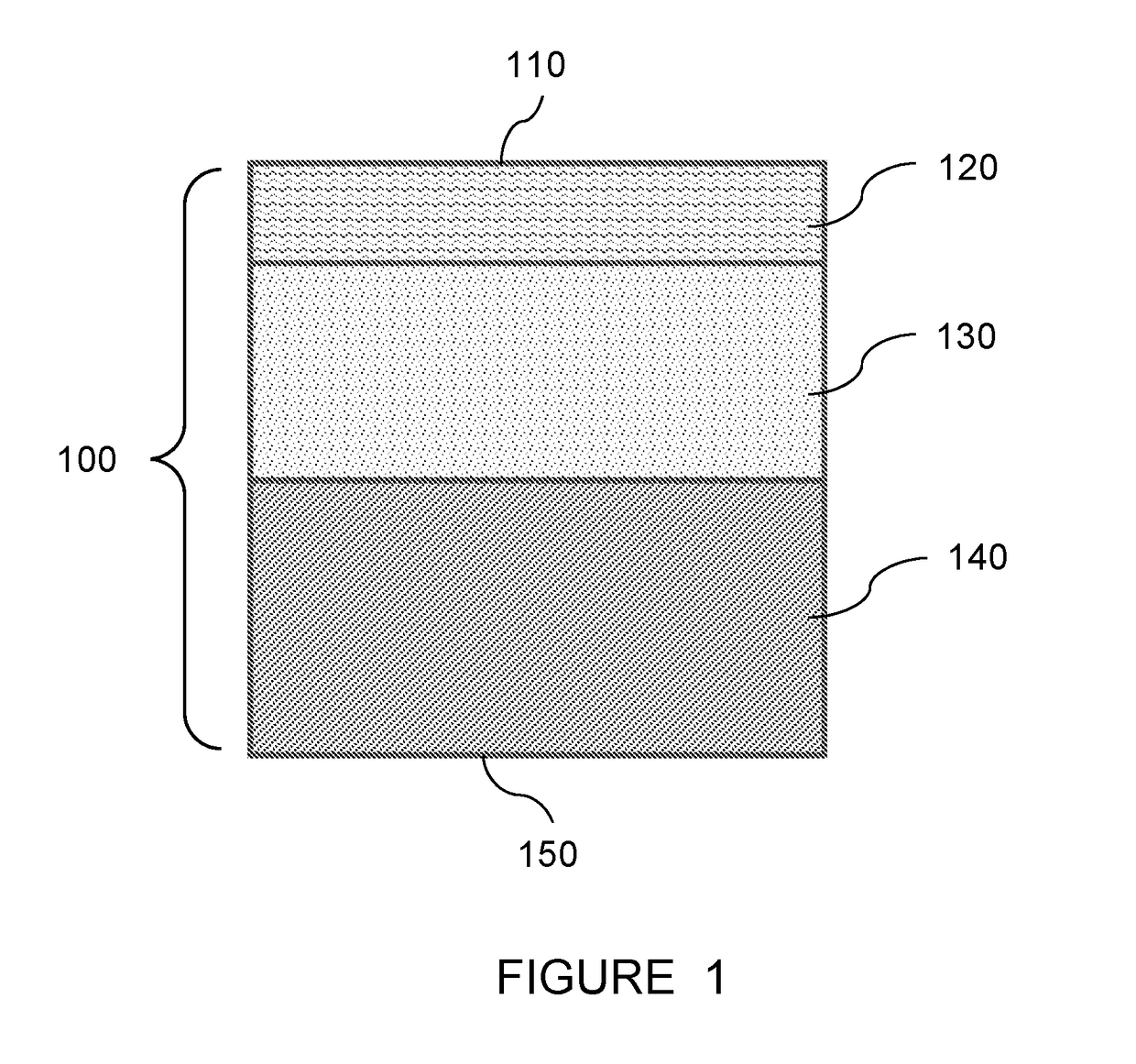
[0089]An aqueous phase was prepared. The aqueous phase contained 4.5 wt % triethylammonium camphorsulfonate (TEACSA, Sunland Chemicals, Los Angeles, Calif.) and 4 wt % m-phenylene diamine (MPD, Dupont, Wilmington, Del.). The aqueous phase was prepared by first adding the DI water to a mixing vessel, followed by addition of the TEACSA and MPD, although any order of addition can be used.
[0090]An organic phase was prepared. The organic phase solution contained 0.2 wt % TMC (Sigma Aldrich, St. Louis, Mo.) in an isoparafinnic solvent, Isopar™ G solvent (a low odor, low aromatic hydrocarbon solvent from ExxonMobile Chemical Company, Houston, Tex.). The organic phase was prepared by placing the Isopar G in a vessel and mixing in the TMC.
Membrane Formation
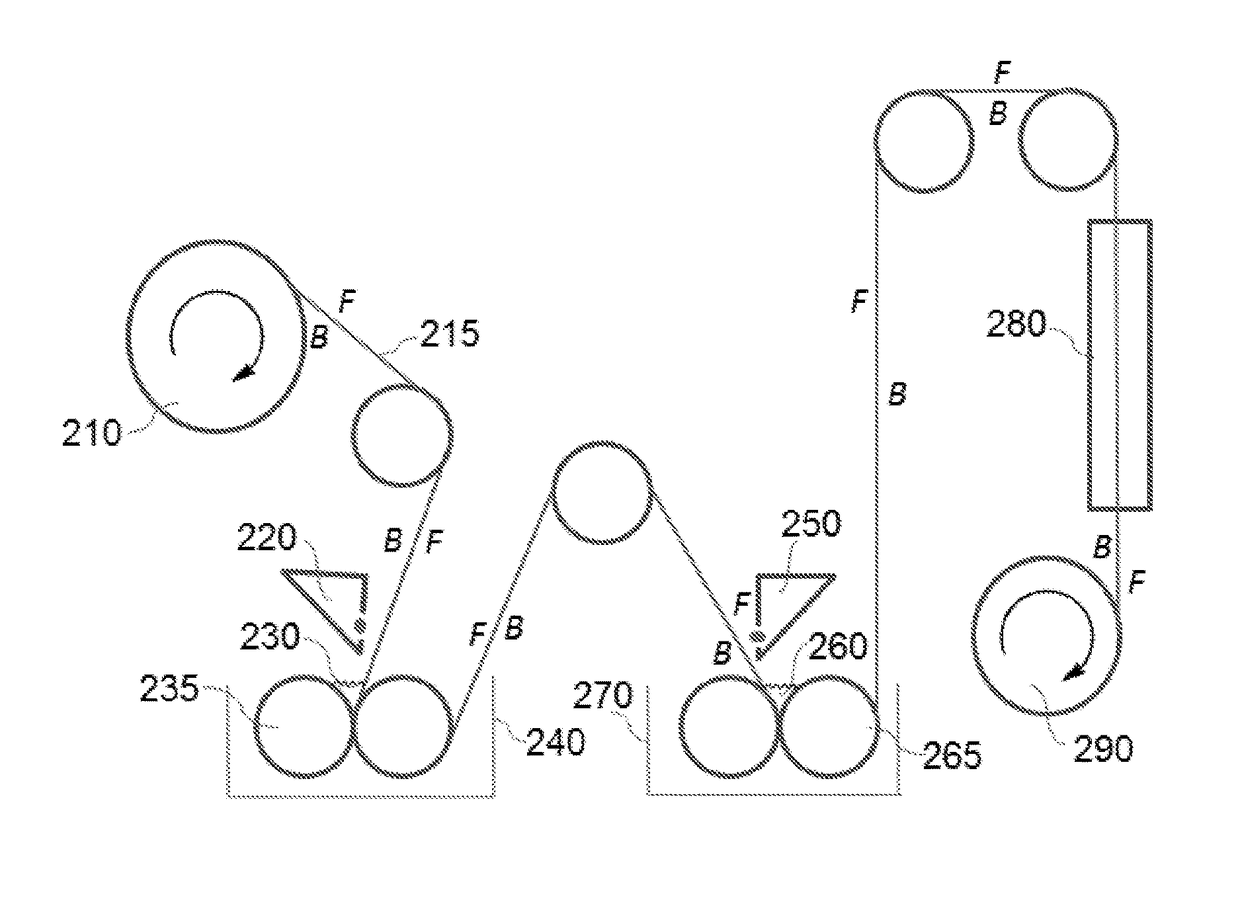
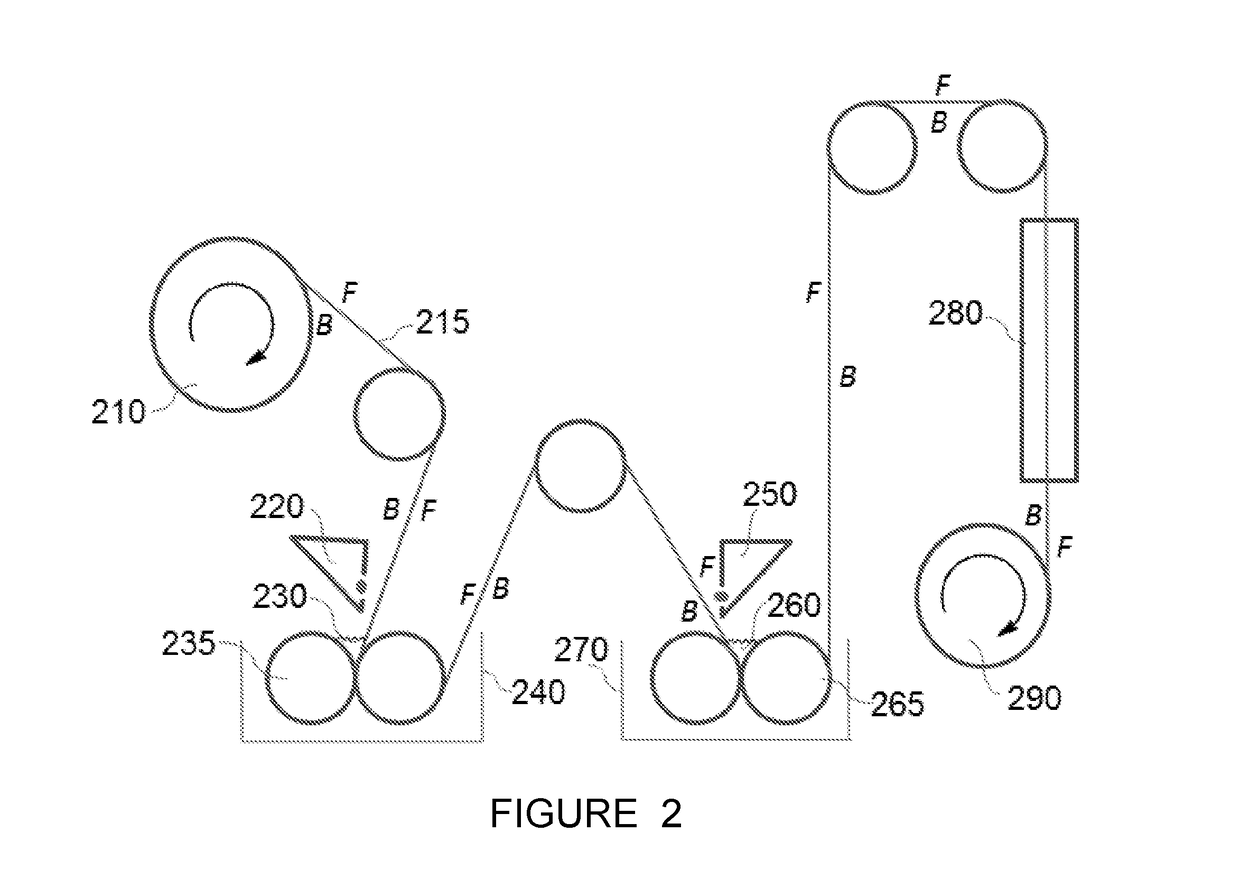
[0091]A polyester non-woven reinforced polysulfone support was used. The aqueous phase was applied to the polysulfone support at ambient temperature (25° C.) and pressure (1 atm). After 10 seconds, any exce...
example 2
Preparation of Brackish Water Membrane
[0092]An aqueous phase was prepared. The aqueous phase contained 6.75 wt % triethylammonium camphorsulfonate (TEACSA, Sunland Chemicals, Los Angeles, Calif.) and 3.5 wt % m-phenylene diamine (MPD, Dupont, Wilmington, Del.). The aqueous phase was prepared by first adding the DI water to a mixing vessel, followed by addition of the TEACSA and MPD, although any order of addition can be used.
[0093]An organic phase was prepared. The organic phase solution contained 0.25 wt % TMC (Sigma Aldrich, St. Louis, Mo.) in an isoparafinnic solvent, Isopar™ G solvent (a low odor, low aromatic hydrocarbon solvent from ExxonMobile Chemical Company, Houston, Tex.). The organic phase was prepared by placing the Isopar G in a vessel and mixing in the TMC.
Membrane Formation
[0094]A polyester non-woven reinforced polysulfone support was used. The aqueous phase was applied to the polysulfone support at ambient temperature (25° C.) and pressure (1 atm). After 10 seconds,...
examples 9 through 16
Varying Amounts of TEACSA in Backside Treatment
[0105]The effect of concentration of the tertiary amine salt in the backside treatment prior to drying on membrane performance was measured. After formation of each of the membranes described in Examples 1 and 2, each membrane was rinsed with 60° C. water several times to eliminate any excess residual compounds in the membrane. Separate membranes of Example 1 and Example 2 were then treated on the backside with solutions of TEACSA of varying concentrations. For the sea water membranes of Example 1, Example 9 was treated with 5 wt % TEACSA, Example 10 was treated with 10 wt % TEACSA, Example 11 was treated with 20 wt % TEACSA, Example 12 was treated with 30 wt % TEACSA, and Example 13 was treated with 40 wt % TEACSA. For the brackish water membranes of Example 2, Example 14 was treated with 5 wt % TEACSA, Example 15 was treated with 10 wt % TEACSA, Example 16 was treated with 20 wt % TEACSA, Example 17 was treated with 30 wt % TEACSA, an...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| pressure | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com