Pharmaceutical Composition for Safe and Effective Treatment of Knee and/or Hip Pain
a technology of drugs and compositions, applied in the field of treating or preventing pain in joints, can solve the problems of adverse central nervous system effects, inability to provide adequate pain relief for inability to meet the needs of patients with acute and chronic pain, so as to reduce pain in the knee joint and reduce arthropathy.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Patients and Methods
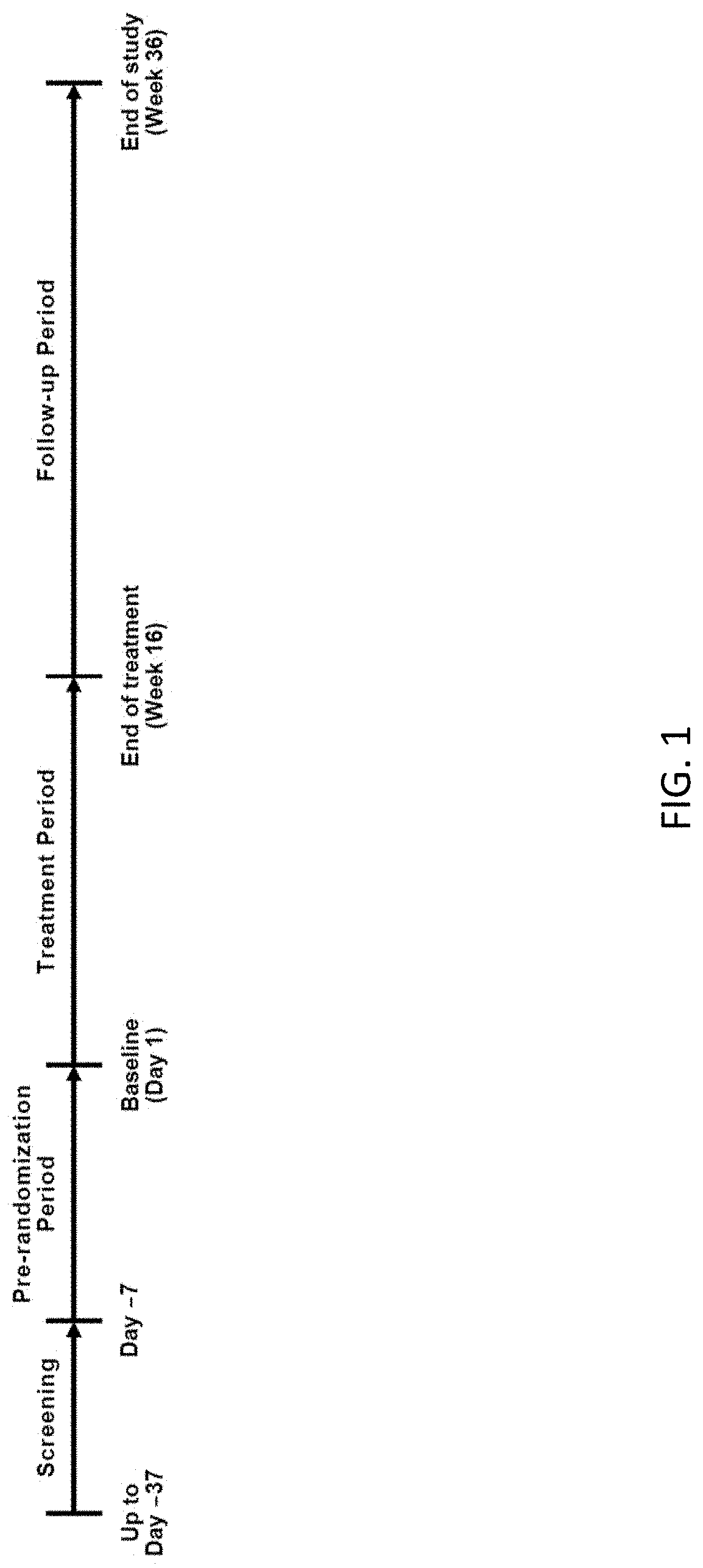
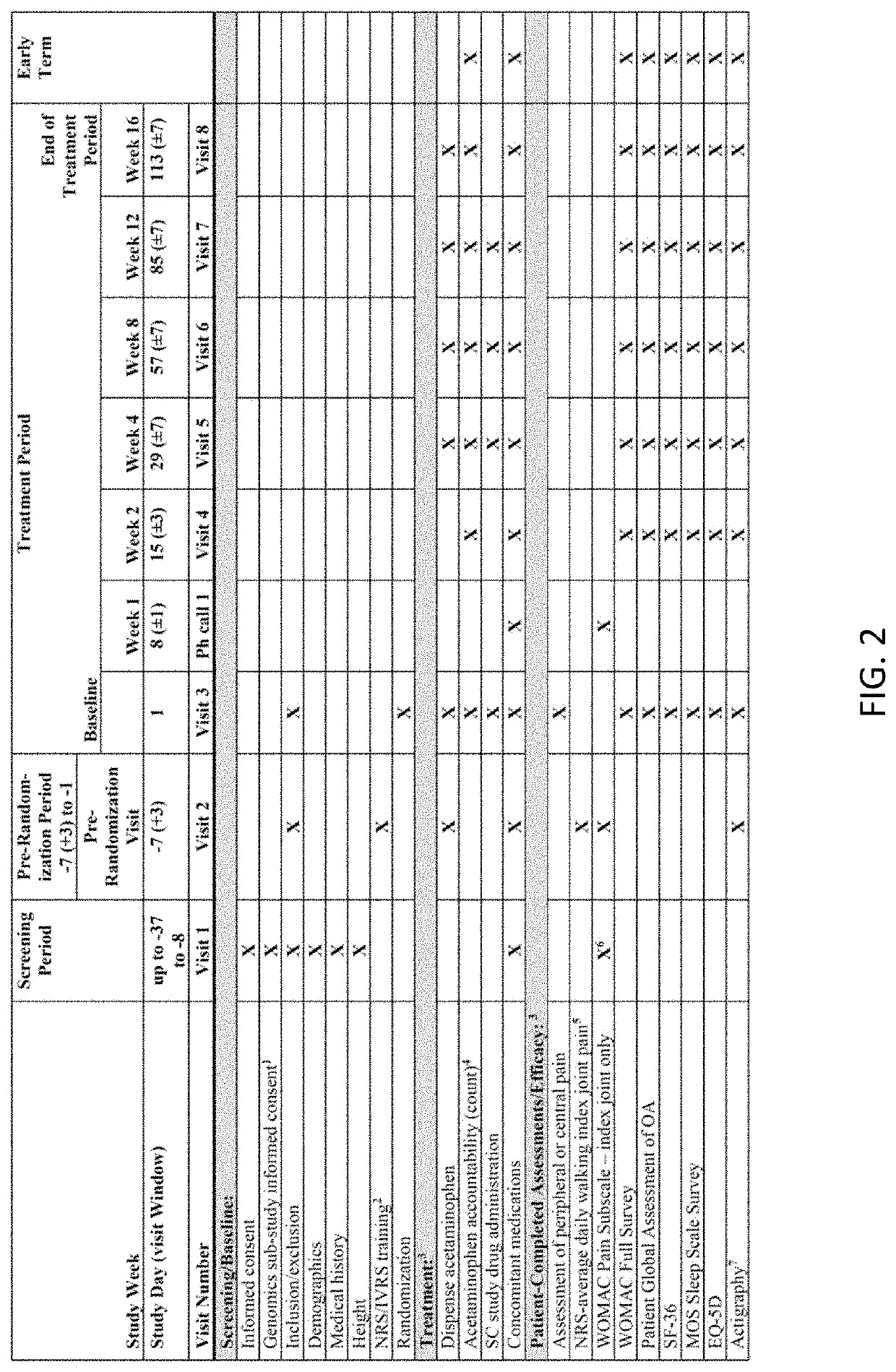
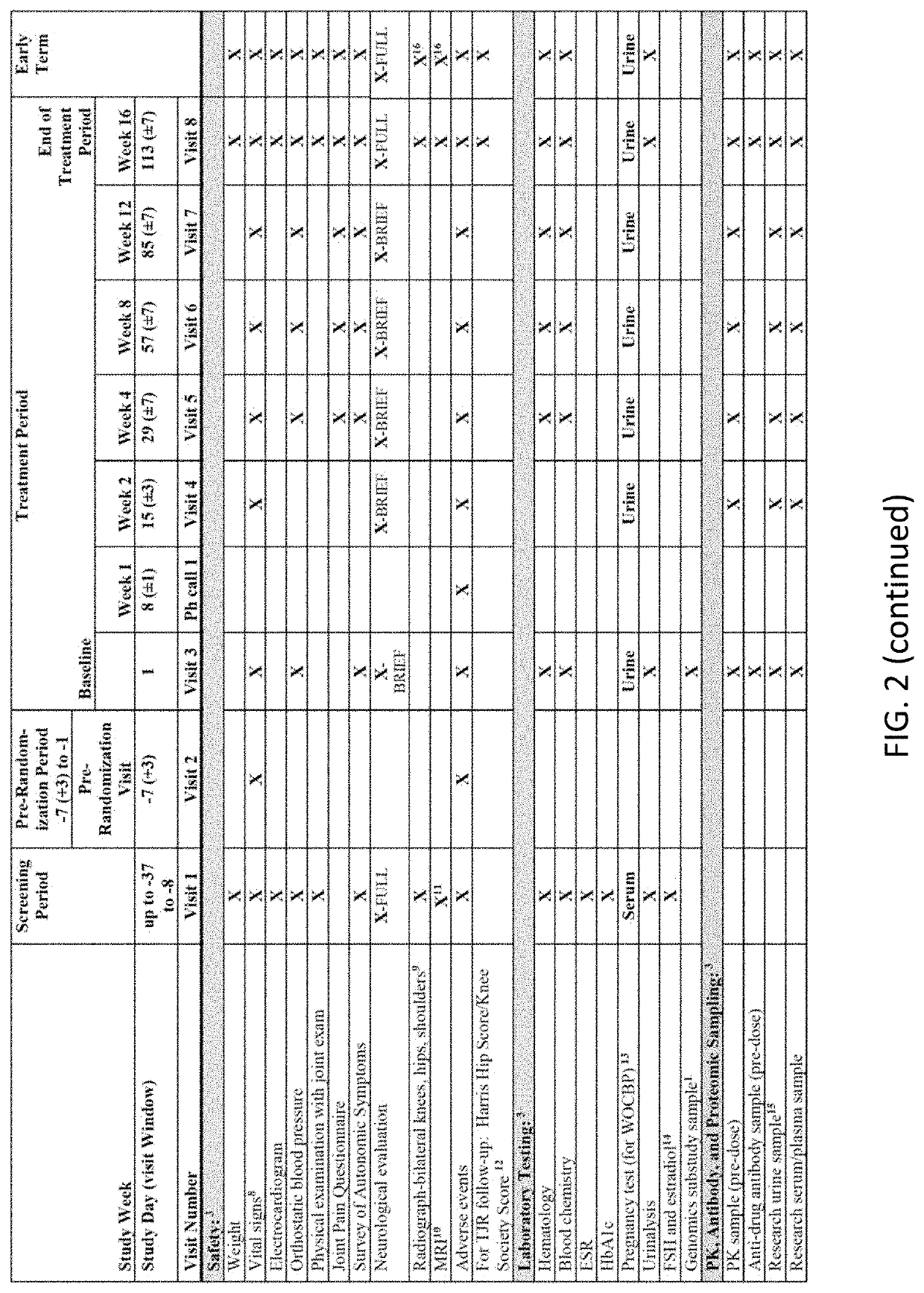
[0206]Patients. Eligible patients were 40-80 years of age; had a diagnosis of OA of the knee and / or hip (designated the most symptomatic, index joint at the time of the screening visit) based on the American College of Rheumatology criteria for OA with radiologic confirmation (Kellgren-Lawrence [K-L] grading of ≥2 on a scale of 0-4); and demonstrated moderate-to-severe pain in the index joint, defined as a Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) pain subscale score of ≥4, at both the screening while on usual analgesic medication and at the randomization visit, which occurred 7 days after withdrawal of analgesic therapy. Patients were required to have a history of inadequate pain relief with or intolerance to acetaminophen and ≥1 oral NSAID, and a history of inadequate pain relief with, intolerance to, or unwillingness to use opioids. Patients were also required to have a history of regular analgesic use for OA pain (average of 4 day...
PUM
| Property | Measurement | Unit |
|---|---|---|
| physical function | aaaaa | aaaaa |
| affinity | aaaaa | aaaaa |
| frequency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com