Infigratinib for treatment of fgfr3-related skeletal diseases during pregnancy
a technology of fgfr3 and infigratinib, which is applied in the direction of skeletal disorders, drug compositions, medical preparations, etc., can solve the problems of defective development and growth of the mandibl
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example
Introduction
[0020]FGFR3-related chondrodysplasias encompass the most frequent chondrodysplasias (achondroplasia and hypochondroplasia) and Craniosynostoses (Muenke syndrome). All these osteochondrodysplasias are due to Fgfr3 germinal mutations, these Fgfr3 gain-of-function mutations impair the chondrogenesis and osteogenesis during the formation of the skeleton.
[0021]Objective of the Study:
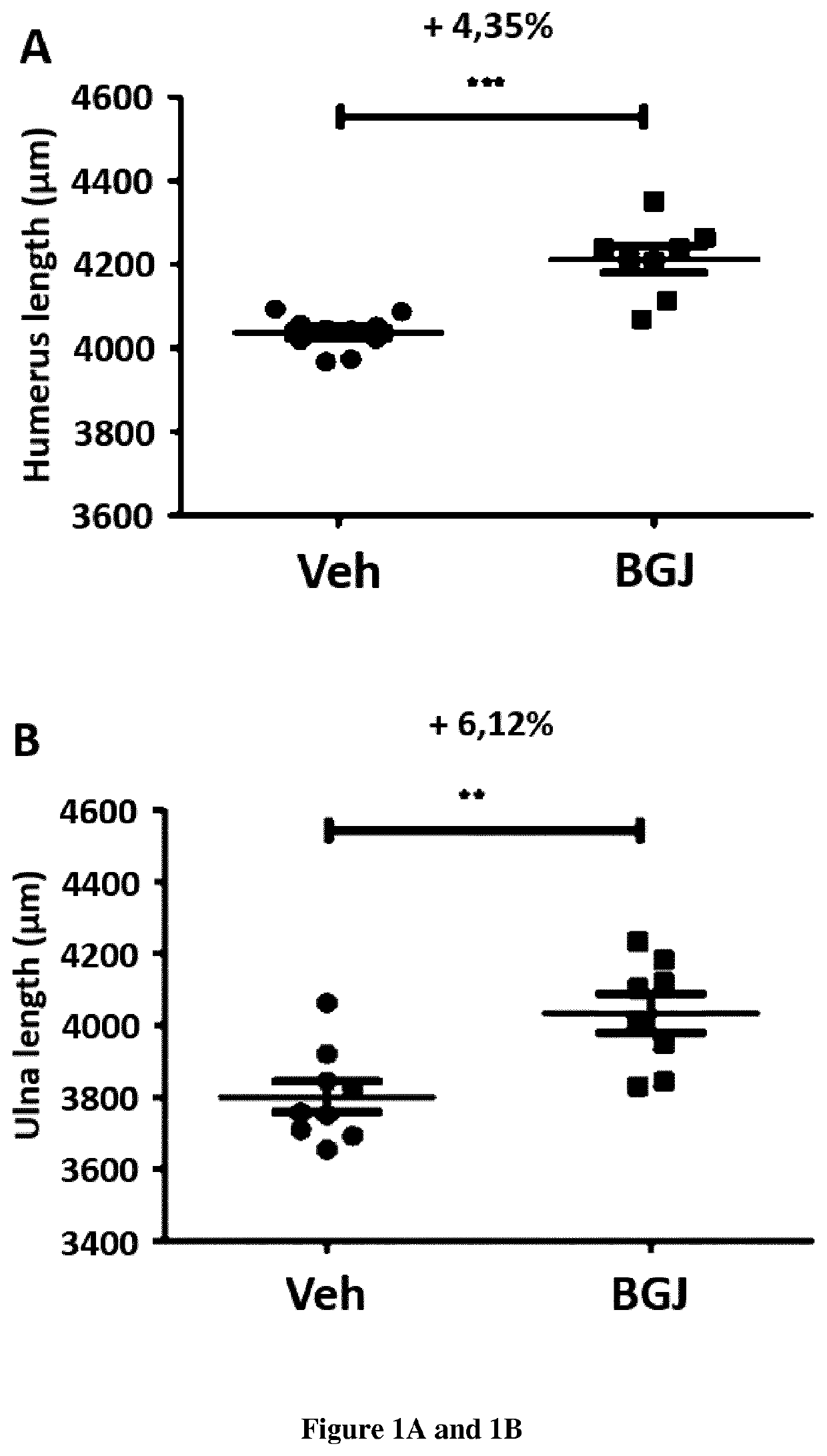
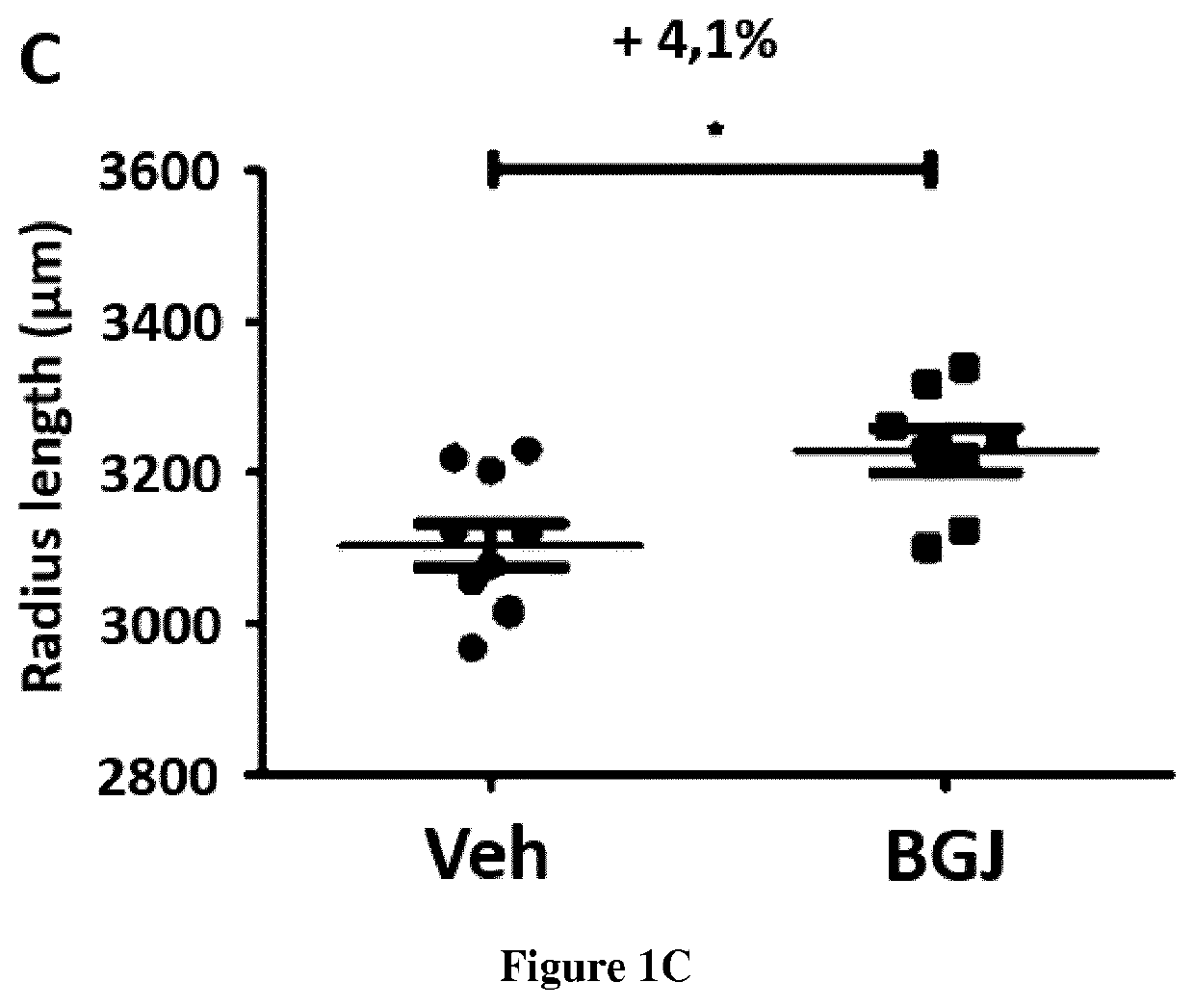
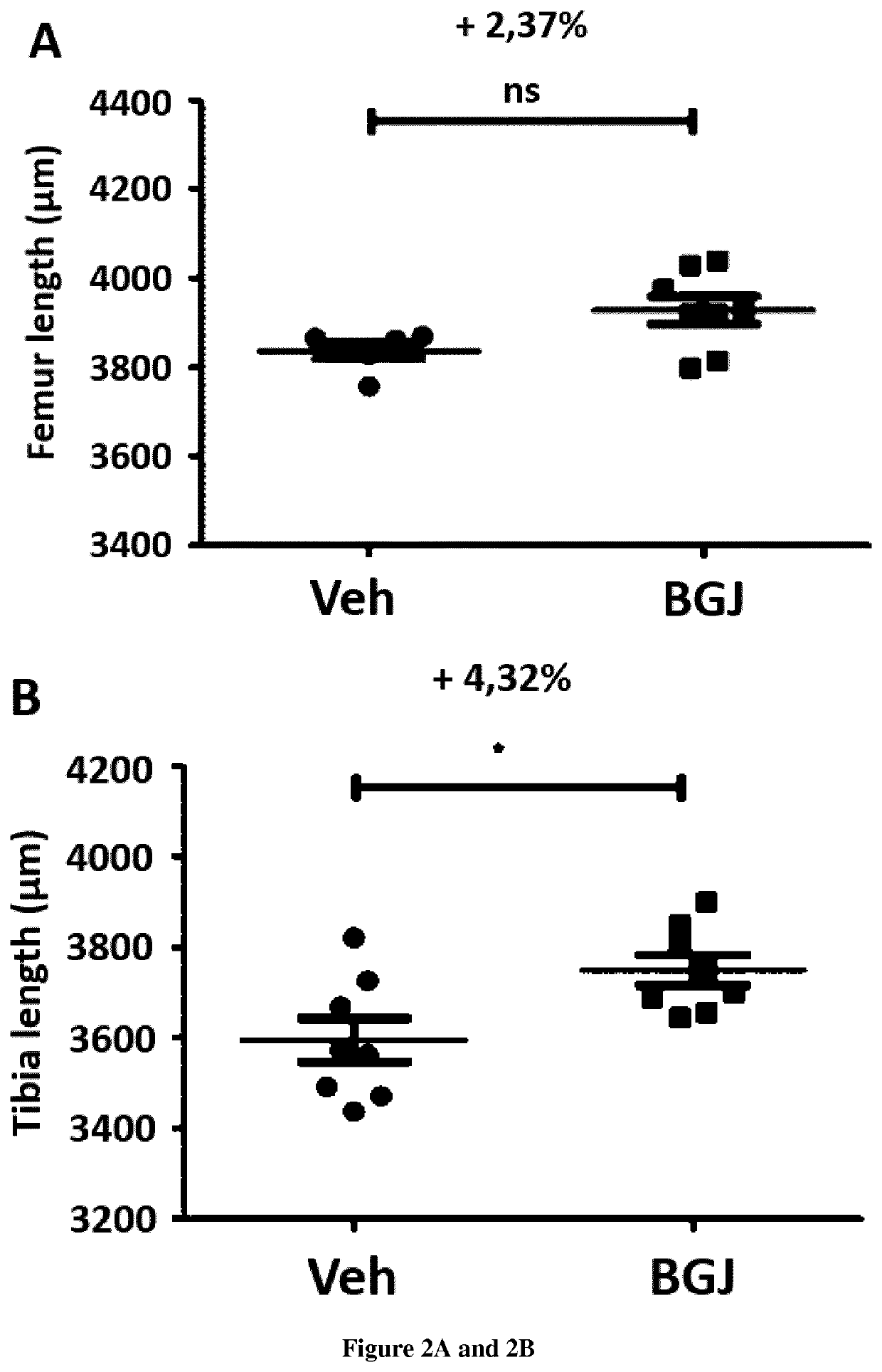
[0022]To demonstrate that it is feasible to treat with the BGJ398 the defective growth of the skeleton during the pregnancy.
[0023]Materials and Methods:
[0024]The efficacy of BGJ398 treatment on skeletal development was demonstrated previously on Fgfr3Y367C / + pups exhibiting dwarfism (Komla-Ebri et al 2016, Biosse-Duplan et al 2016).
[0025]We treated pregnant female Fgfr3Neo / Y367C mice that were mated with CreCMV / + male mice (Pannier, S., Couloigner, V., Messaddeq, N., Elmaleh-Bergès, M, Munnich, A., Romand, R., & Legeai-Mallet, L. (2009). Activating Fgfr3 Y367C mutation causes hearing loss and inne...
PUM
| Property | Measurement | Unit |
|---|---|---|
| width | aaaaa | aaaaa |
| ultrasound evaluation | aaaaa | aaaaa |
| time | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com