Compositions and methods for intravitreal delivery of polynucleotides to retinal cones
a technology of polynucleotide and retinal cone, which is applied in the field ofviral-based gene therapy of retinal disorders, can solve problems such as the risk of creating additional damage to retinal tissu
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Background
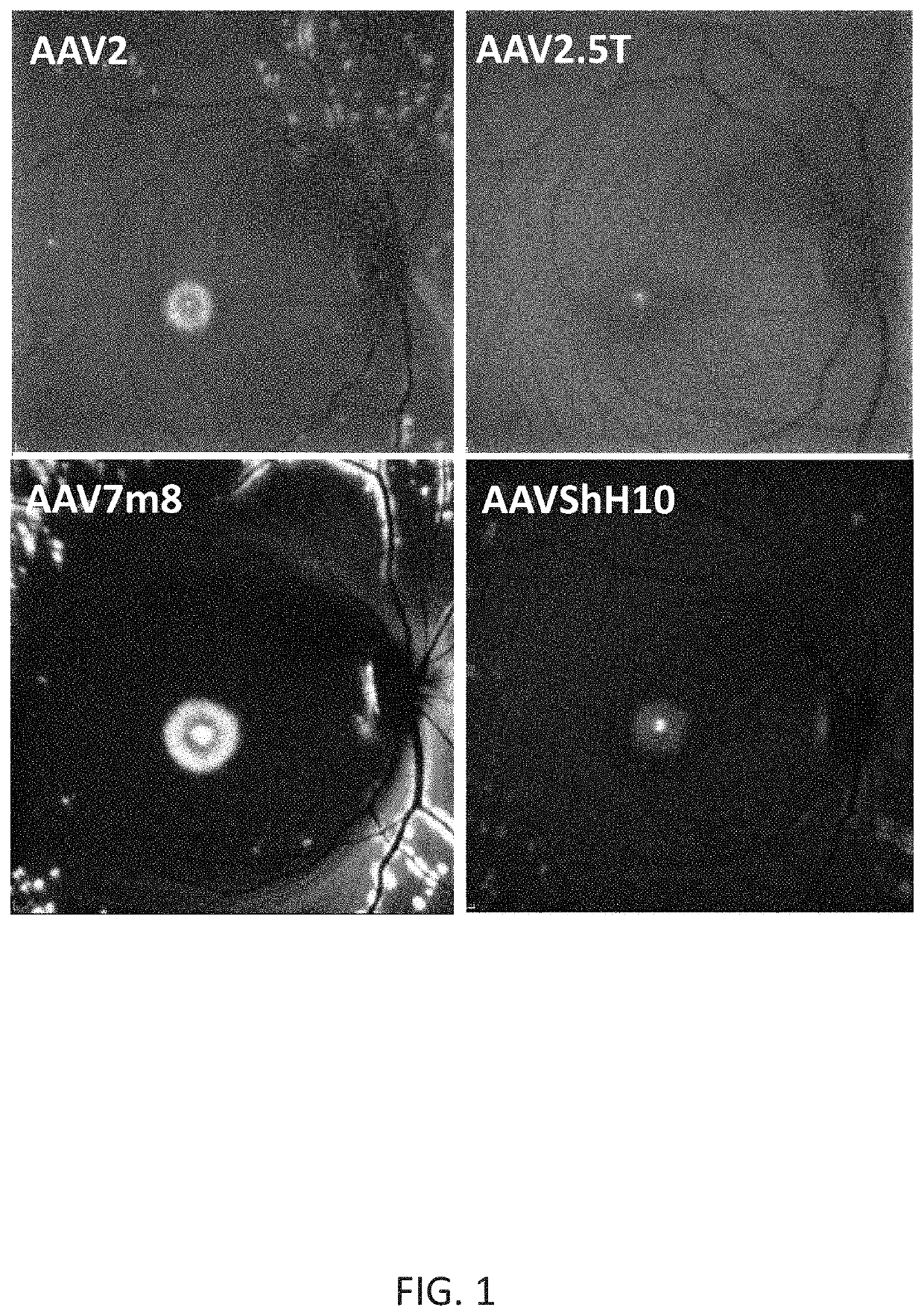
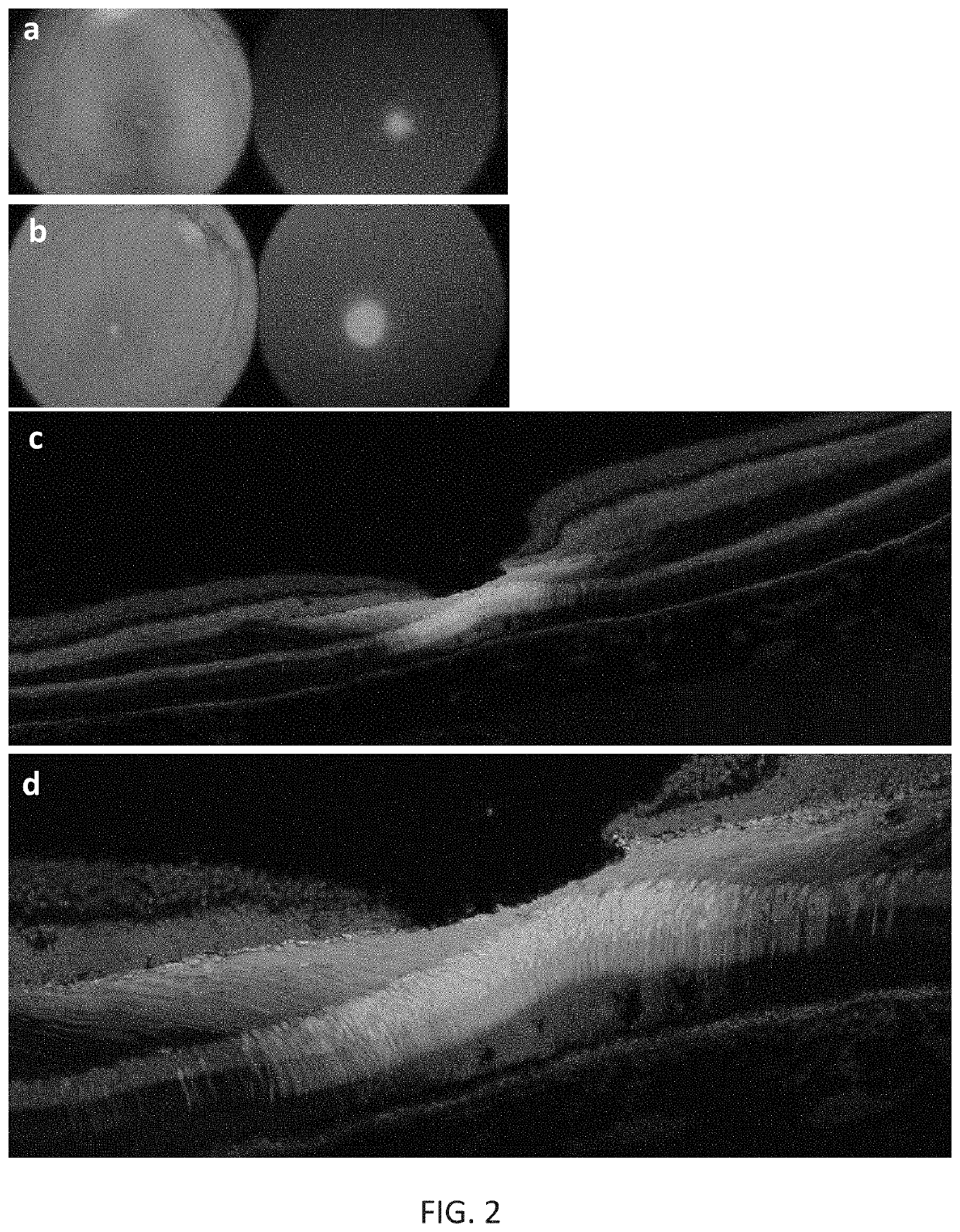
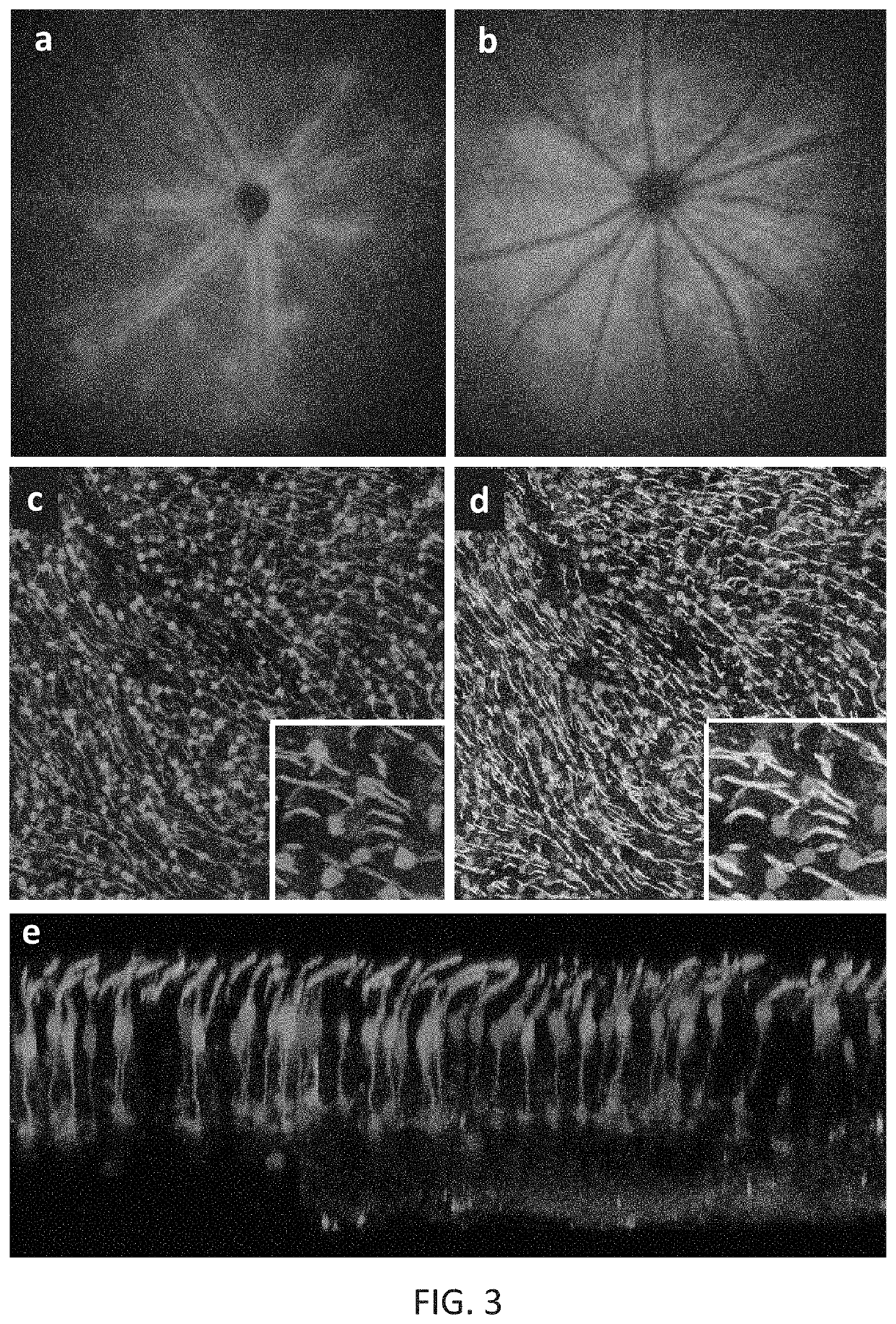
[0174]New therapies are needed for the treatment of many cone photoreceptor associated disorders, including macular dystrophies such as cone-rod dystrophy, cone dystrophy, Stargardt macular dystrophy, and achromatopsia; color vision disorders such as protan, deutan, and tritan defects; and vision disorders of the central macula such as age-related macular degeneration, macular telangiectasia, retinitis pigmentosa, diabetic retinopathy, retinal vein occlusions, glaucoma, Sorsby's fundus dystrophy, adult vitelliform macular dystrophy, Best's disease, and X-linked retinoschisis. As these vision disorders are associated with a loss of function and / or viability of the cone photoreceptors, it is hypothesized that these disorders may be treatable by delivering a therapeutic gene to cone photoreceptors to rescue cone viability and function.
[0175]To that end, the polynucleotide cassette “pMNTC” was designed in which enhancer, promoter, 5′UTR, intron, Kozak, and polyadenylation sequ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com