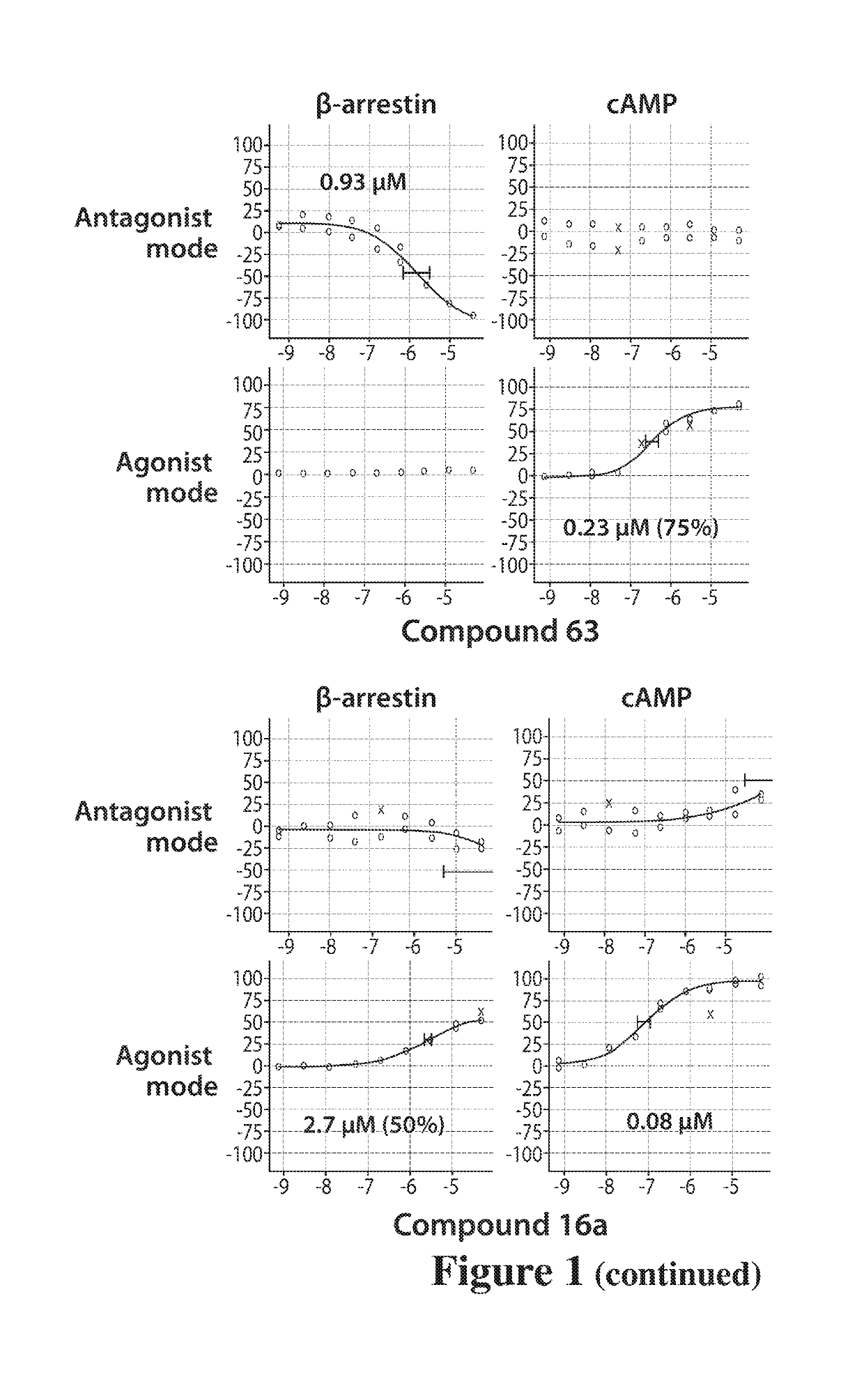
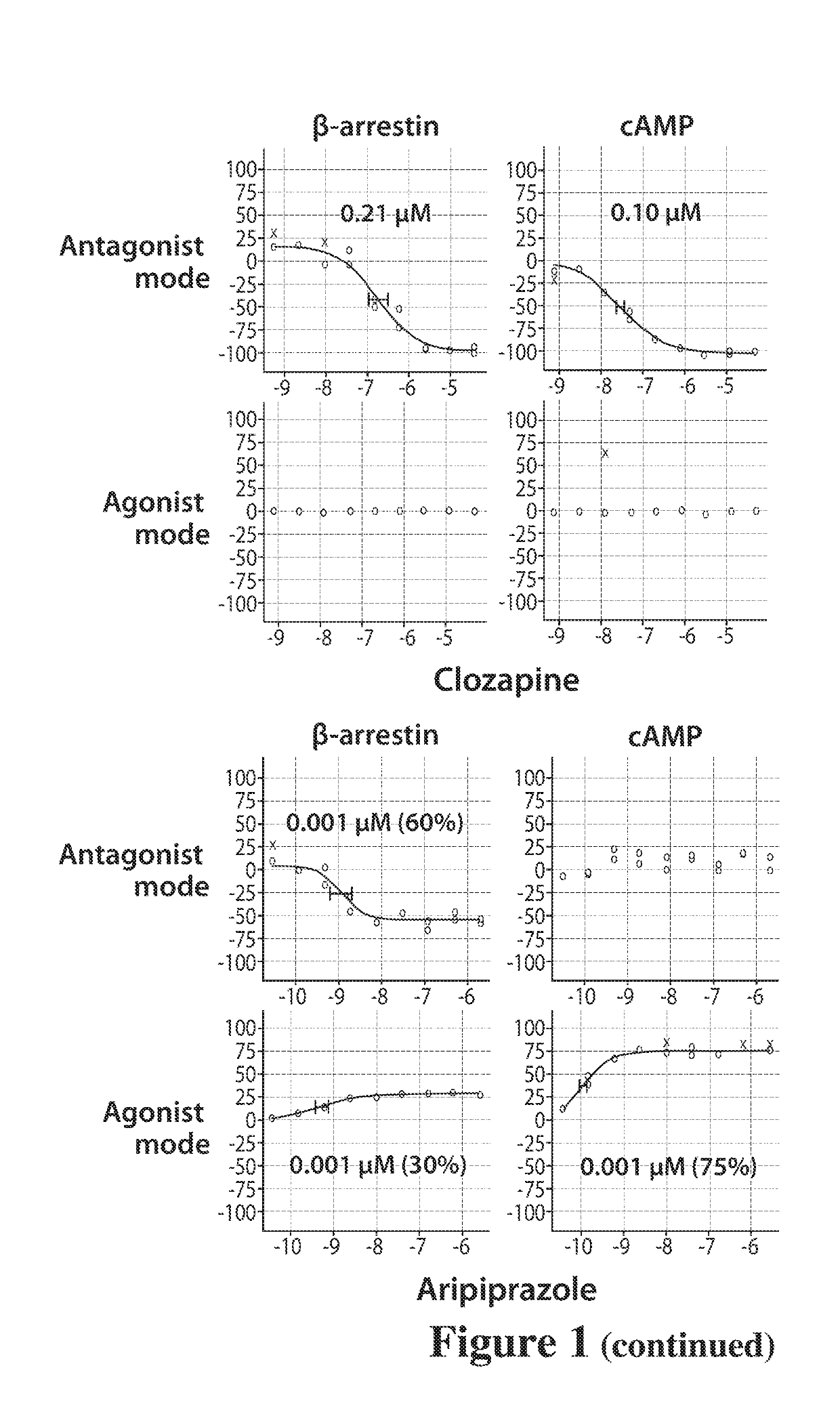
Dopamine d2 receptor ligands
a technology ligand, which is applied in the field of ligands of dopamine d2 receptor, can solve the problems of undesirable side effects and lack of clinical drug candidates that offer highly functionalized, and achieve the effects of minimizing potential associated side effects, minimizing potential undesirable side effects, and more selectivity and functionality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
examples
Synthesis of Intermediate I
[0561]
Step 1: (4-Chlorophenyl)(pyridin-2-yl)methanol
[0562]
[0563]To a stirred suspension of magnesium (5.37 g, 223.75 mmol, 3 equiv) in dry THF (60 mL) under argon atmosphere was added iodine (2 crystals), 1,2-dibromo ethane (2 drops). 1-bromo-4-chlorobenzene (25.76 g, 134.39 mmol, 1.8 equiv) was then added dropwise for 1 h at room temperature. The reaction mixture was stirred at room temperature for 1 h. A solution of picolinaldehyde (8 g, 74.68 mmol) in dry THF (19 mL) was added drop wise at room temperature and stirred for 2 h. After completion of reaction, the reaction mixture was quenched with saturated ammonium chloride solution and extracted with EtOAc. The combined organic extract was washed with water, brine, dried over sodium sulfate, filtered and concentrated under reduced pressure. Purification using silica gel column chromatography (40% EtOAc / hexanes as eluent) afforded 12.26 g of (4-chlorophenyl) (pyridin-2-yl) methanol (yield=75%). ESI+MS: m / ...
example-1
1-(2-(3-chlorophenoxy)ethyl)-N-((4-chlorophenyl)(pyridin-2-yl)methyl)piperidine-4-carboxamide (I)
[0701]
[0702]To a stirred solution of N-((4-chlorophenyl)(pyridin-2-yl)methyl)piperidine-4-carboxamide (Int-I) (0.20 g, 0.60 mmol) in CH3CN (5 mL) were added potassium carbonate (0.251 g, 1.81 mmol, 3 equiv) and 1-(2-bromoethoxy)-3-chlorobenzene (0.143 g, 0.60 mmol, 1 equiv) at room temperature. The reaction mixture was heated at 80° C. and stirred for 6 h. After completion, the reaction mixture was diluted with water and extracted with CH2Cl2. The combined organic extract was dried over sodium sulfate, filtered and concentrated under reduced pressure. Purification using silica gel column chromatography (3% MeOH / CH2Cl2 as eluent) afforded 0.05 g of 1-(2-(3-chlorophenoxy) ethyl)-N-((4-chlorophenyl) (pyridin-2-yl) methyl) piperidine-4-carboxamide (Yield=17%).
[0703]1H NMR (400 MHz, DMSO-d6): δ 8.71(d, 1H, J=6.4 Hz), 8.51(d, 1H, J=4.0 Hz), 7.78-7.76 (m, 1H), 7.44 (d, 1H, J=8.0 Hz), 7.37-7.25 ...
example-2
1-(2-(4-chlorophenoxy)ethyl)-N-((4-chlorophenyl)(pyridine-2-yl)methyl) piperidine-4-carboxamide (2)
[0704]
[0705]Title compound was prepared from N-((4-chlorophenyl) (pyridin-2-yl) methyl) piperidine-4-carboxamide (Int-I) (0.20 g, 0.60 mmol) using the general methodology of Example-1. The product was washed with n-pentane and ether and afforded 0.1 g of 1-(2-(4-chlorophenoxy) ethyl)-N-((4-chlorophenyl) (pyridine-2-yl) methyl) piperidine-4-carboxamide (Yield=34%).
[0706]1H NMR (400 MHz, DMSO-d6): δ 8.70 (d, 1H, J=8.0 Hz), 8.51 (d, 1H, J=4.0 Hz), 7.80-7.75 (m, 1H), 7.44 (d, 1H, J=8.0 Hz), 7.37-7.35 (m, 7H), 6.96 (d, 2H, J=8.8 Hz), 6.13 (d, 1H, J=8.4 Hz), 4.05 (bs, 2H), 2.92 (bs, 2H), 2.65 (bs, 2H), 2.33-2.32 (m, 1H), 2.01 (bs, 2H), 1.66-1.55 (m, 4H); ESI+MS: m / z: 484.5 ([M+H]+).
PUM
| Property | Measurement | Unit |
|---|---|---|
| time | aaaaa | aaaaa |
| time | aaaaa | aaaaa |
| time period | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com