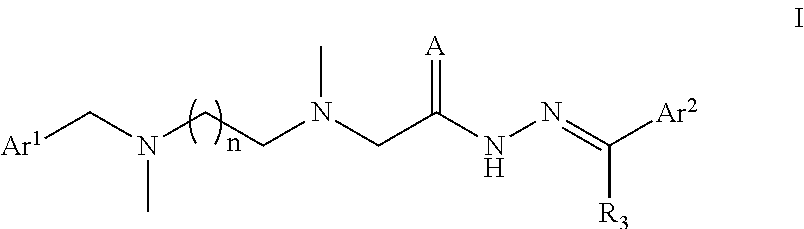
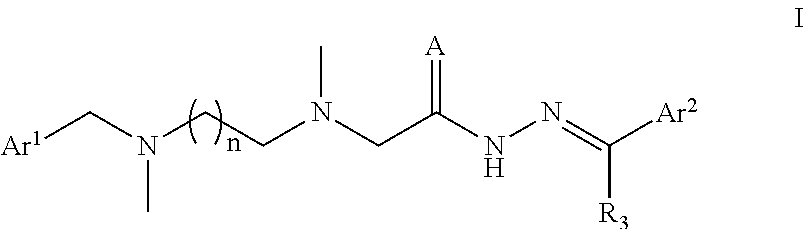
A Substituted Acethydrazide Derivative, Preparation Method and Use Thereof
a technology of acethydrazide and derivative, applied in the field of substituted acethydrazide derivative, preparation method, etc., can solve the problem of hindering degradation of acethydrazid
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
preparation example 1
[0072]
[0073]9.6 mL (0.36 mol) of N,N′-dimethylethanediamine and 100 mL of tetrahydrofuran were added to a three-necked bottle equipped with constant pressure funnel, reflux condensing tube and thermometer, mixed evenly and then heated. When the reaction solution was slightly boiled (about 56° C.), 10 g (0.06 mol) of 1-chloromethylnaphthalene was slowly added in dropwise to the reaction solution, white precipitate was gradually generated, and thin-layer chromatography (TLC) was used to monitor the end of reaction. When the spot of 1-chloromethylnaphthalene in the reaction solution disappeared on thin-layer chromatography, heating was stopped, cooling was carried out, solvent was dried out by rotary evaporation, 3 mol / L of sodium hydroxide solution was used for washing, dichloromethane was used for extracting the washing liquid in which pH≧12 was maintained during extraction, all organic phases were combined, separated by silica column (eluent: dichloromethane / methanol=20 / 1) to Obtain...
preparation example 3
[0076]
[0077]2.1 g (0.06 mol) of 85% hydrazine hydrate and 50 ml of anhydrous ethanol were added to 250 mL three-necked bottle equipped with constant pressure funnel, reflux condensing tube and thermometer, mixed evenly and then heated to reflux, 5.0 g (0.02 mol) of methyl 2-{N-{2-[N-(naphthalen-1-yl-methylene)-N-methylamino]ethyl}N-methylamino}acetate was slowly added to the reaction solution, and reacted for 2 h, then heating was stopped, cooling was carried out, solvent was dried out by rotary evaporation, silica column separation was carried out (eluent: dichloromethane / methanol=10 / 1) to obtain 2-{N-{2-[N-(naphthalen-1-yl-methylene)-N-methylamino]ethyl}N-methylamino}acethydrazide, white solid, yield 86%. 1H-NMR (400 MHz, CDCl3) δ: 8.18 (7H, m), 4.10 (2H, s), 3.29 (2H, s), 2.37 (4H, m), 2.26 (6H, s); EI-MS (m / z): 301.2 [M+H]+.
preparation example 4
[0078]
[0079]Pare- and meta-trifluoromethylbenzyl chloride was used as raw material, and operations were the same for Intermediate 1. Light yellow liquid product was obtained. MS[M]+=246.1 m / e.
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| HIF-α | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com