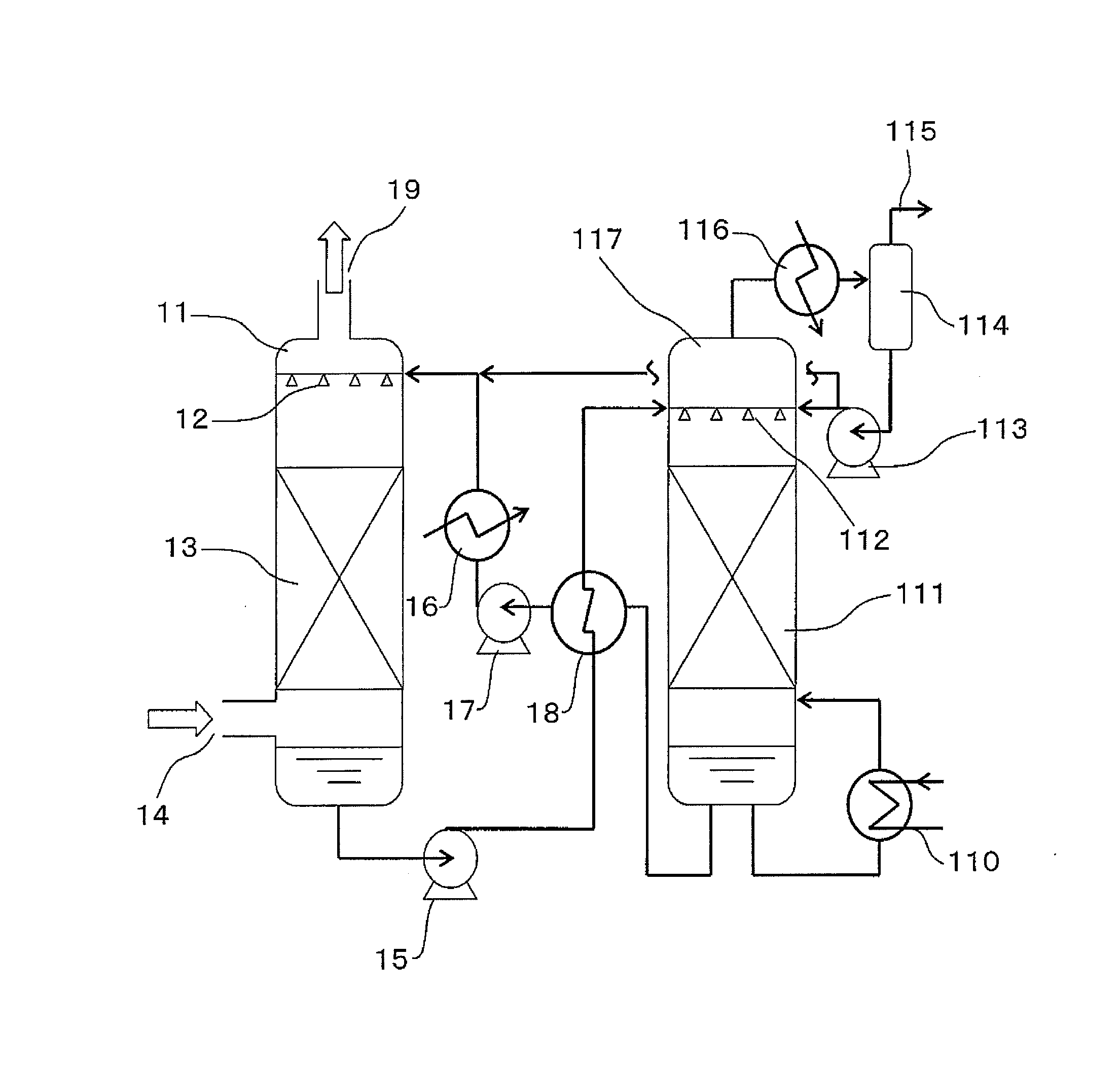
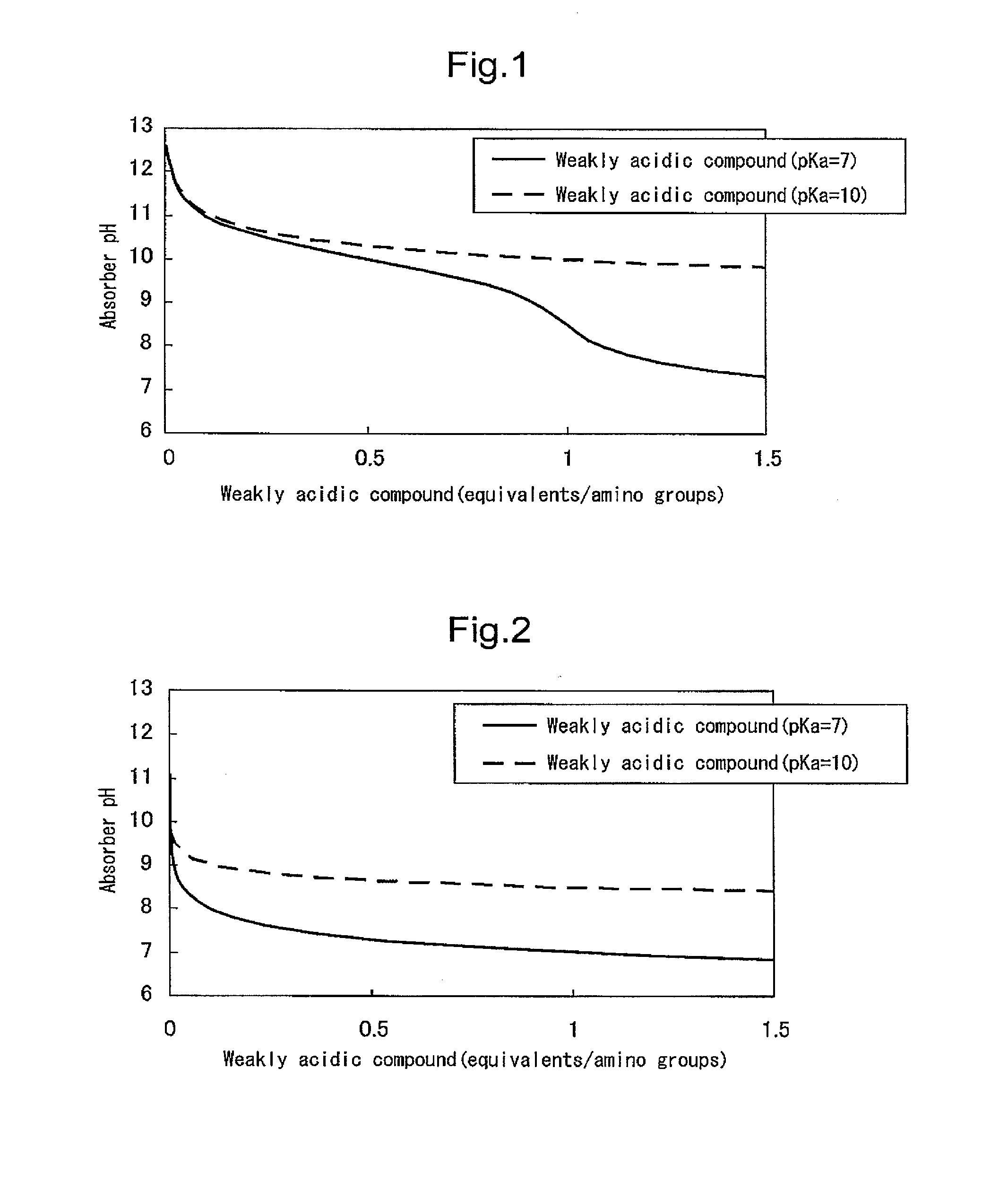
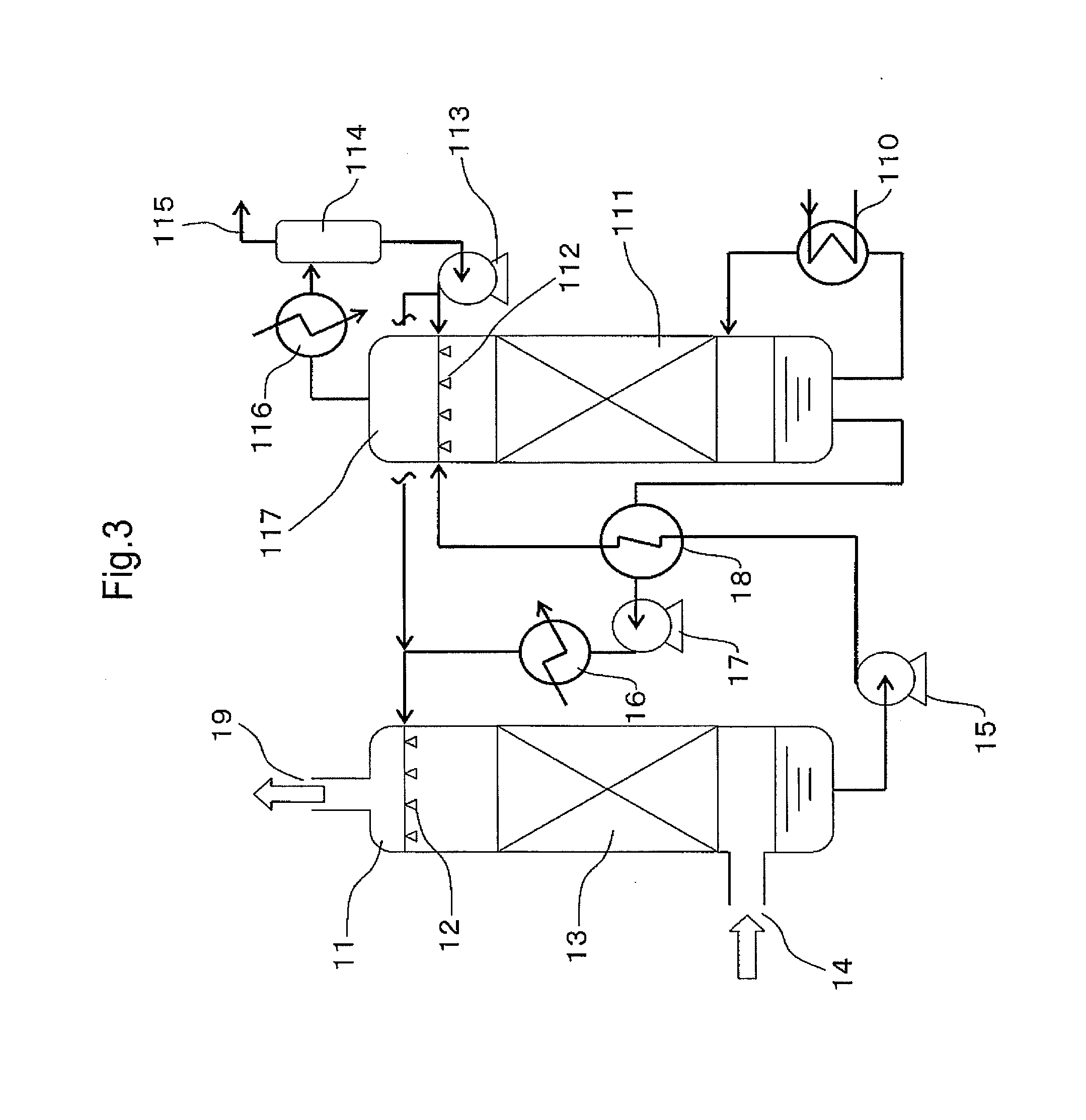
Carbon dioxide absorber and carbon dioxide separation/recovery method using the absorber
a carbon dioxide and absorber technology, applied in the direction of hydrogen sulfides, separation processes, energy input, etc., can solve the problems of difficult to save energy, serious economic shortcomings, and inability to know the chemical properties of absorption of carbon dioxide, so as to prevent volatilization of amine compounds and metal corrosion, reduce the effect of oxidation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
examples
[0182]The following provides a more detailed explanation of the present invention through examples thereof. Furthermore, the present invention is not limited by the following examples.
[0183]
[0184]MEA: Monoethanolamine
[0185]DEA: Diethanolamine
[0186]Pz: Piperazine
[0187]MDEA: Methyldiethanolamine
[0188]BHEP: 1,3-bis(2-hydroxyethylamino)propan-2-ol
[0189]BHPP: 1,3-bis(2-hydroxypropylamino)propan-2-ol
[0190]HEHPP: 1-(2-hydroxyethylamino)-3-(2-hydroxypropylamino)propan-2-ol
[0191]DAP: 1,3-diaminopropan-2-ol
[0192]THPP: 1,3-tetrakis(2-hydroxypropylamino)propan-2-ol
[0193]EAE: 2-ethylaminoethanol
[0194]2A13PD: 2-amino-1,3-propanediol
[0195]IPAE: 2-isopropylaminoethanol
[0196]TMB: Trimethylborate
[0197]TEB: Triethylborate
Carbon Dioxide Absorber of First Configuration
examples 1 to 4
[0198]In order to evaluate the first configuration of the present invention, an amine compound, a weakly acidic compound and water were mixed and dissolved in the ratios shown in Table 1 to obtain carbon dioxide absorbers. The pKb values of the amine compounds, the pKa values of the weakly acidic compounds, and the number of equivalents of the weakly acidic compounds with respect to amino groups are shown in Table 1. The pKb values of the amine compounds and the pKa values of the weakly acidic compounds were respectively calculated from the pH value in a 0.4 M aqueous solution.
TABLE 1WeaklyEq / no.WaterAmineWtacidicWtof aminocontentcompoundpKb%compoundpKa%groups(wt %)Ex. 1MEA4.728.3Boric acid9.25.70.266.0Ex. 2MEA4.723.0Boric acid9.223.31.053.7Ex. 3DEA5.447.2Boric acid9.25.60.247.2Ex. 4DEA5.445.6Boric acid9.22.30.07*148.9Pz4.83.2Ex. 5Pz4.821.2Boric acid9.215.20.563.6Comp. Ex. 1MEA4.728.3Acetic acid4.85.60.266.1Comp. Ex. 2MEA4.730.0None———70.0Comp. Ex. 3DEA5.450.0None———50.0Comp. Ex. 4D...
examples 6 to 12
[0222]In order to evaluate the first configuration of the present invention, an amine compound, a weakly acidic compound and water were mixed and dissolved in the ratios shown in Table 4 to obtain carbon dioxide absorbers. The results of testing these absorbers in the same manner as the absorbers of Examples 3 and 4 and Comparative Examples 3 to 5 are shown in Table 5.
TABLE 4WeaklyEq / no.WaterAmineWtacidicWtof aminocontentcompoundpKb%compoundpKa%groups(wt %)Ex. 6EAE4.444.0Boric acid9.230.153.0Ex. 72A13PD5.645.0Boric acid9.230.152.0Ex. 8IPAE4.150.0Boric acid9.230.147.0Ex. 9IPAE4.145.5Boric acid9.23.50.1*246.5Pz4.846.6Ex. 10MDEA5.746.6Boric acid9.22.50.08*347.5Pz4.83.4Ex. 11DEA5.450.0TMB8.74.80.0945.2Ex. 12DEA5.450.0TEB8.16.80.0943.2Comp. Ex. 7EAE4.444.0None———56.0Comp. Ex. 82A13PD5.645.0None———55.0Comp. Ex. 9IPAE4.150.0None———50.0Comp. Ex. 10IPAE4.145.5None———50.0Pz4.84.5Comp. Ex. 11MDEA5.746.6None———50.0Pz4.83.4*20.9 equivalents when converted based on 1 mole of piperazine*31.0 equiv...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pKa | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| pKa | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com