Medicinal composition for promoting synthesis of protoporphyrin ix
a technology of protoporphyrin and composition, which is applied in the direction of drug compositions, antibacterial agents, peptide/protein ingredients, etc., can solve the problems that the effective method of treating infection by mdrp has not yet been established, and achieves the effects of preventing the emergence of mdrp in the patient, preventing the onset of mdrp, and reducing the risk of infection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0084]Multi-drug resistant Pseudomonas aeruginosa (MDRP: ATCC BAA-2110) was placed in tryptic soy broth medium (TBS: from DIFCO), and cultured at 37° C. for 24 hours. With an absorptiometer, the TBS medium comprising the cultured MDRP was adjusted to 1×109 CFU (Colony forming unit) / ml. In a 12-well plate, the following solutions (1)-(3) were each prepared and cultured. (1): TBS medium comprising MDRP adjusted to 1×109 CFU / ml, (2): a solution of (1) and ALA added to a final concentration of 0.5 mg / ml, and (3): a solution of (1) and ALA added to a final concentration of 0.5 mg / ml and EDTA added to a final concentration of 400 mg / ml. The solutions were collected 4 hours after the start of culture, and suspended in 0.25 ml of PBS per 1 g of the pelleted bacteria. To 0.2 ml of the suspension, 0.01 ml of 50% acetic acid and 0.9 ml of DMF / IPA (100:1) were added, vortexed, and separated by centrifugation (14, 000 rpm, 5 min, 4° C.). The concentrations of coproporphyrinogen III (CPIII) and p...
example 2
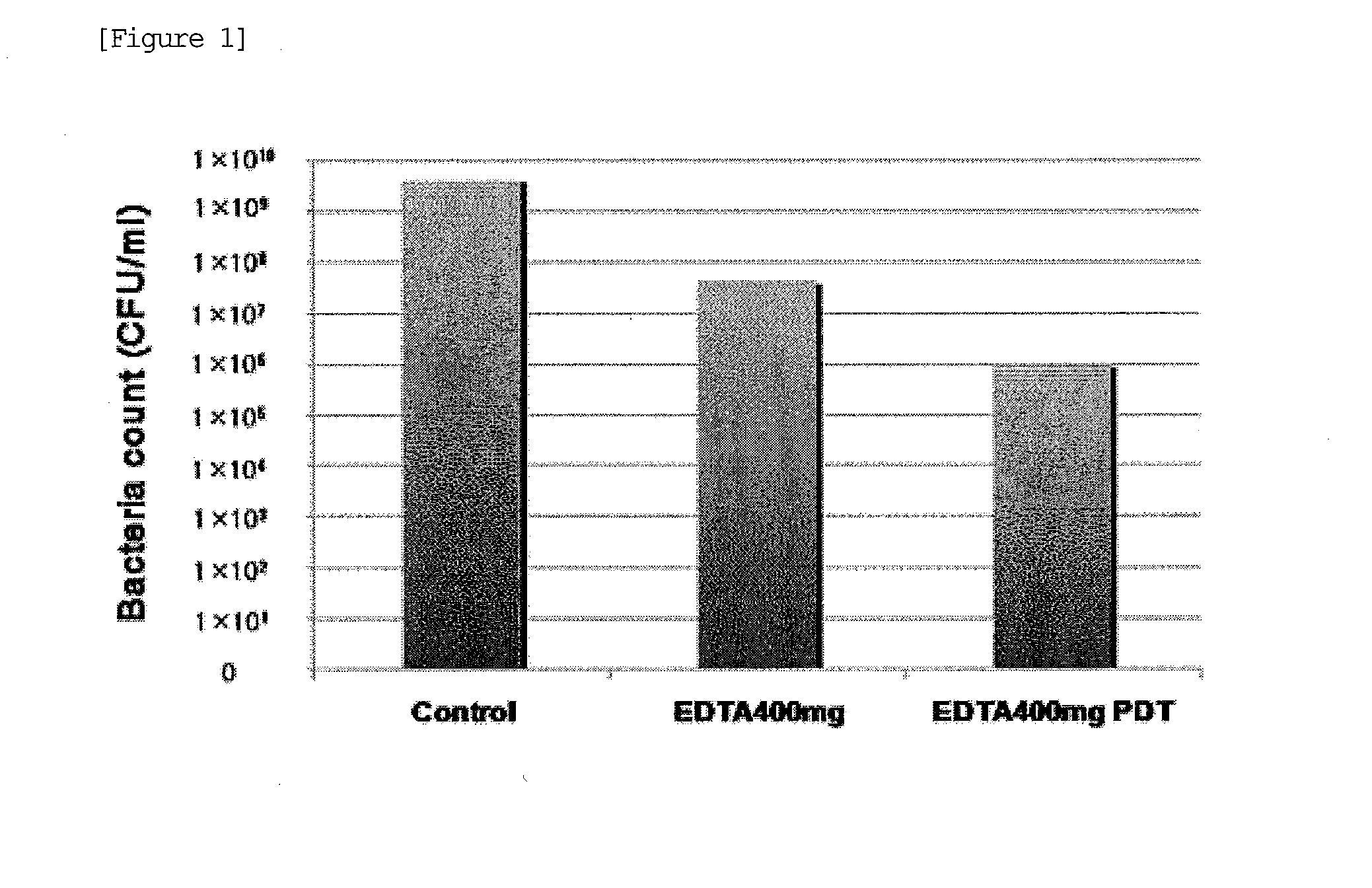
[0087]Multi-drug resistant Pseudomonas aeruginosa (MDRP: ATCC BAA-2110) was cultured for 24 hours at 37° C. with tryptic soy broth medium (TBS: from DIFCO). Then, the TBS medium comprising MDRP at 1×109 CFU / ml was dispensed in a 12-well plate at 1 ml per well, and ALA was added to a final concentration of 1 mg / ml and EDTA was added to a final concentration of 400 mg / ml. These were divided into the group subjected to PDT (PDT group) and the group not subjected to PDT (non-PDT group) (each n =1). For the control group, only ALA at a final concentration of 1 mg / ml was added to 1 ml of the TBS medium comprising MDRP at 1×109 CFU / ml, and PDT was not carried out. The control group and the non-PDT group were cultured for 18 hours, and the number of bacteria in each well was counted. The PDT group was cultured for 18 hours, irradiated with LED (410 nm) at 50 J / cm2, and then the number of bacteria was counted.
[0088]As shown in FIG. 1 and Table 2, the number of bacteria in the control group a...
example 3
[0089]Multi-drug resistant Pseudomonas aeruginosa (MDRP: BAA-2110) was cultured in tryptic soy broth medium (TBS: from DIFCO) for 24 hours at 37° C. Next, the TBS medium comprising MDRP was dispensed in a 12-well dish at 1 ml per well (1×104−2×105 CFU / mL). ALA at a final concentration of 1 mg / ml and a chelating agent other than EDTA (2.5 mg / mL of citric acid, 2.5 mg / mL of malic acid, 1 mg / mL of diethylenetriaminepentaacetic acid (DTPA), 1 mg / mL of triethylenetetramine-N,N,N′,N″,N′″,N″′-hexaacetic acid, 1 mg / mL of N-(2-hydroxyethyl) iminodiacetic acid, and 1 mg / mL of deferoxamine mesylate, each at final concentration) were added to each dish. For the control group, only ALA at a final concentration of 1 mg / ml was added to 1 ml of the TBS medium comprising MDRP. Next, these were cultured for 5 hours, and the number of bacteria in each well was counted. The PDT group was cultured for 5 hours, and then irradiated with LED (410nm) at 50 J / cm2, after which the number of bacteria was count...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
| minimal inhibitory concentration | aaaaa | aaaaa |
| minimal inhibitory concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com