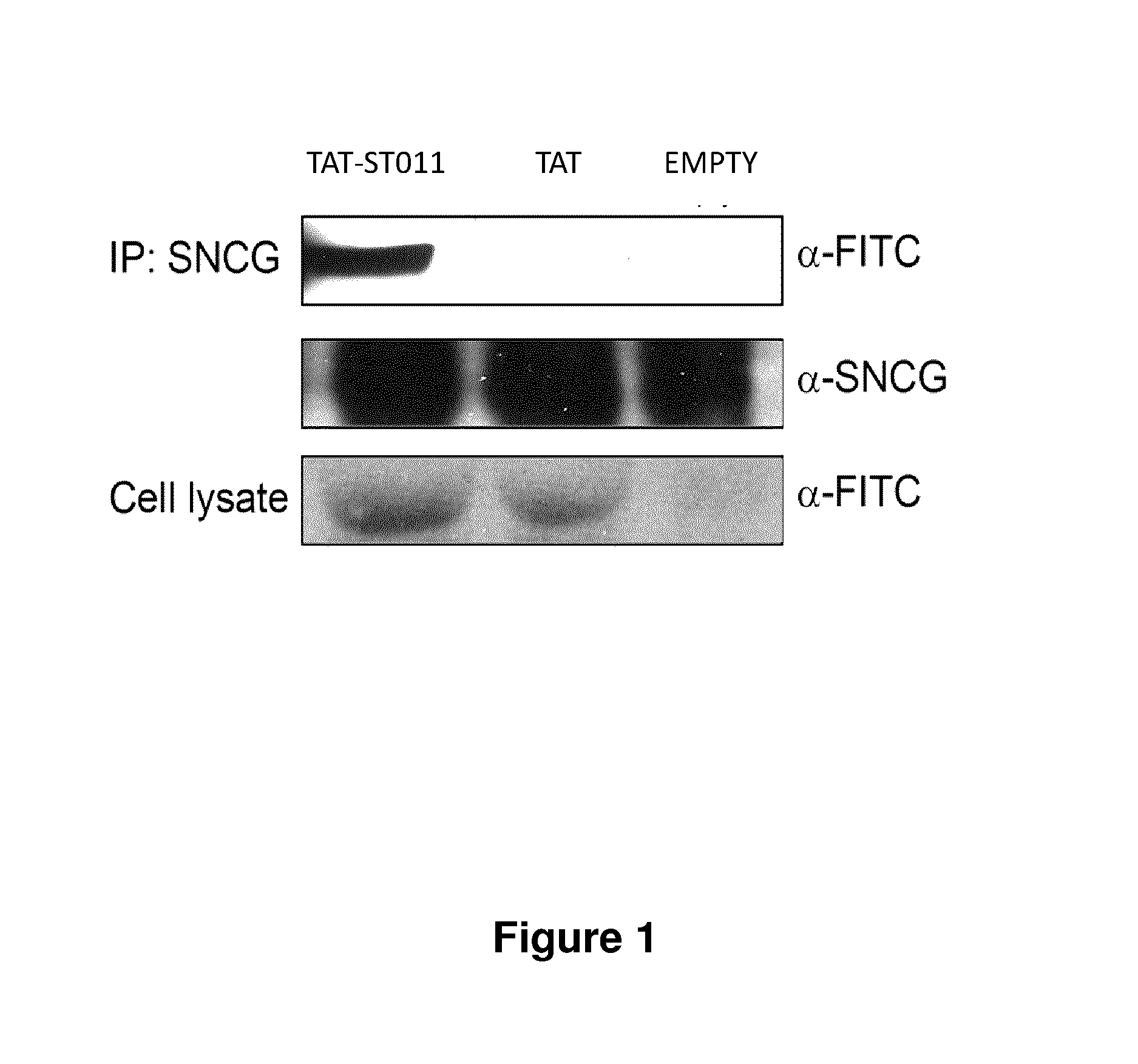
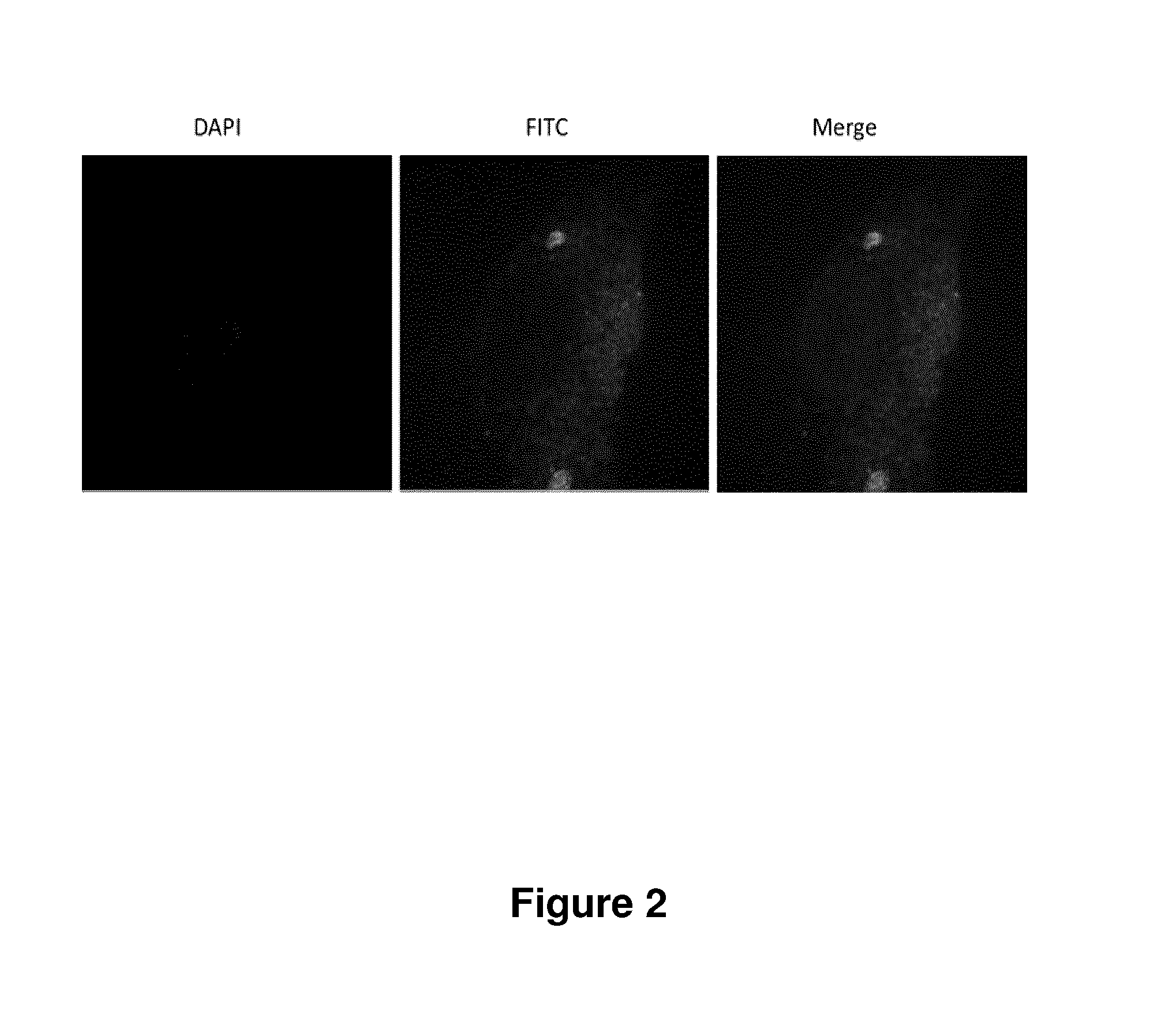
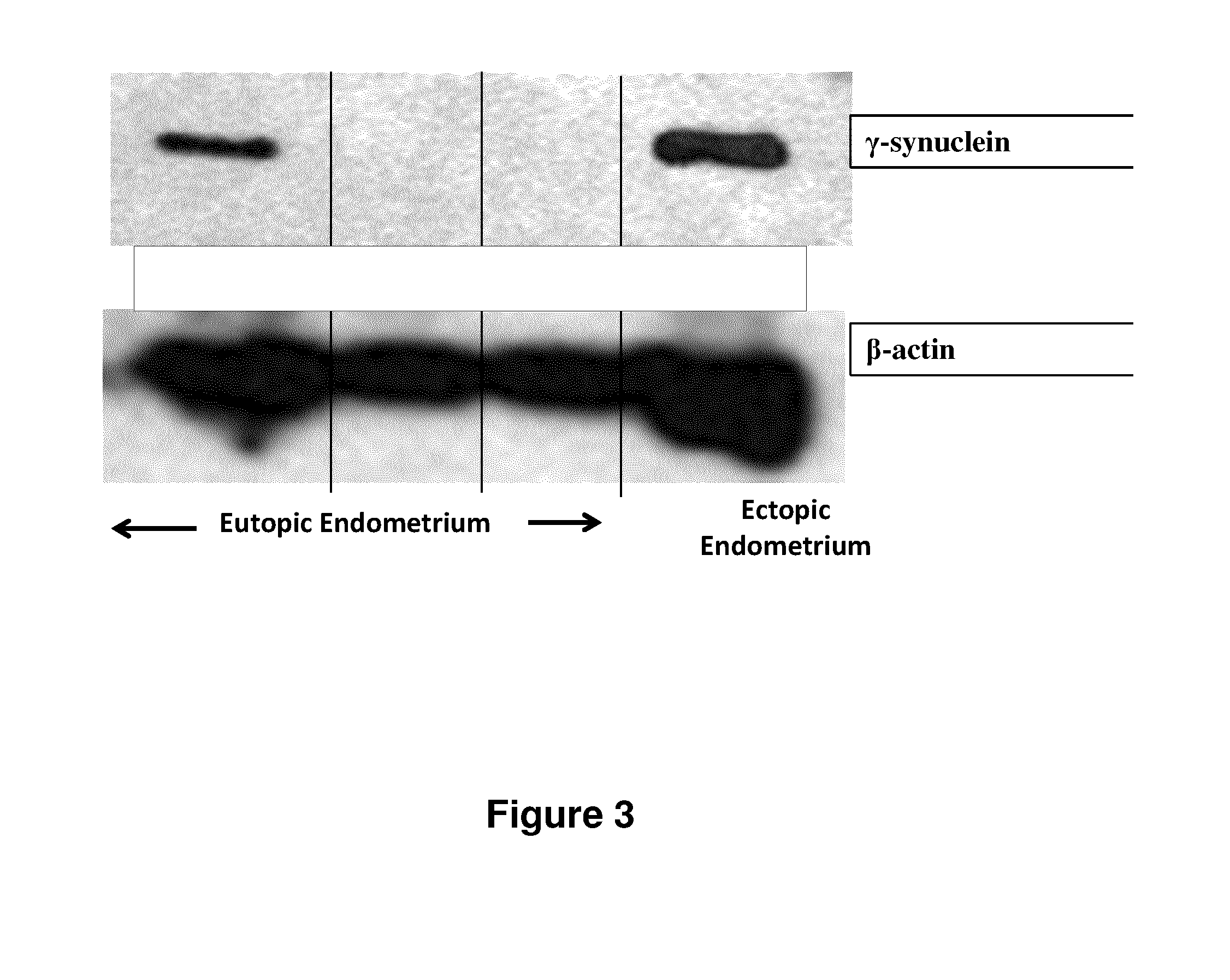
Treatment of endometriosis, angiogenesis and/or endometrial lesion growth
a technology of endometriosis and angiogenesis, applied in the field of treatment of endometriosis, can solve the problems of not being suitable for long-term therapy, not supporting pregnancy, and none of these treatments, so as to reduce the number of available er- receptors and reduce the proliferation of sncg-mediated cells
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Cell Culture
[0096]HUVECs (Cell Applications, San Diego, Calif., USA) obtained previously from umbilical cord veins were cultured and maintained in T75 flasks containing endothelial cell growth media (Cell Applications, San Diego, Calif., USA). Cells were incubated at 37° C. in a humidified chamber with 5% CO2. HUVECs used in assays were between the third and the seventh passage.
[0097]Luminal epithelial cells derived from endometrium adenocarcinomas (CRL-2923, ATCC, Manassas, Va., USA) were cultured and maintained in T75 flasks containing RPMI-1640 (Sigma Chemical Co., St. Louis, Mo., USA) with 5% fetal bovine serum (FBS, Sigma Chemical Co., St. Louis, Mo., USA) and 1% penicillin and streptomycin (Sigma Chemical Co., St. Louis, Mo., USA). These cells were also incubated at 37° C. in a humidified chamber with 5% CO2. Epithelial cells used for assays were between the second and the fourth passage.
example 2
ST011 Peptide Synthesis
[0098]Peptide was synthesized by solid phase synthesis with amino-terminal acetylation and carboxyl-terminal amidation to mimic the intact protein at a commercial facility. Peptide was purified by reverse phase HPLC using a C18 column (Vydac, Hesperia, Calif.) and lyophilized. The molecular mass of the peptide was verified by matrix-assisted laser desorption ionization time-of-flight (MALDI-TOF) mass spectrometry. Fifty μL aliquots of 1.2 mM ST011 peptide in 10 mM sodium phosphate buffer pH 7.0 were stored at −20° C. until use.
example 3
Cell Proliferation Assay
[0099]HUVECs and epithelial cells were trypsinized, suspended in cultured medium and counted. Approximately 10,000 cells per well were seeded onto a 96 well plate. After allowing 24 hours for attachment in 37° C., the cells were treated with either 600 ng / mL, 300 ng / mL, 150 ng / mL, 5 ng / mL or 2.5 ng / mL of TAT-P12 using phosphate buffer saline (PBS, (Sigma Chemical Co., St. Louis, Mo., USA) as a control. The drug was suspended in RPMI. The plates were incubated in 37° C. for 24 or 48 hours after which 10 μL of WST-1(Roche, Laval, QC, Canada) was added to each well. The plate was then incubated in 37° C. for another 4 hours. The absorbance of the wells was measured at a wavelength of 490 nm or at 510 nm.
PUM
| Property | Measurement | Unit |
|---|---|---|
| pH | aaaaa | aaaaa |
| concentrations | aaaaa | aaaaa |
| concentrations | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com