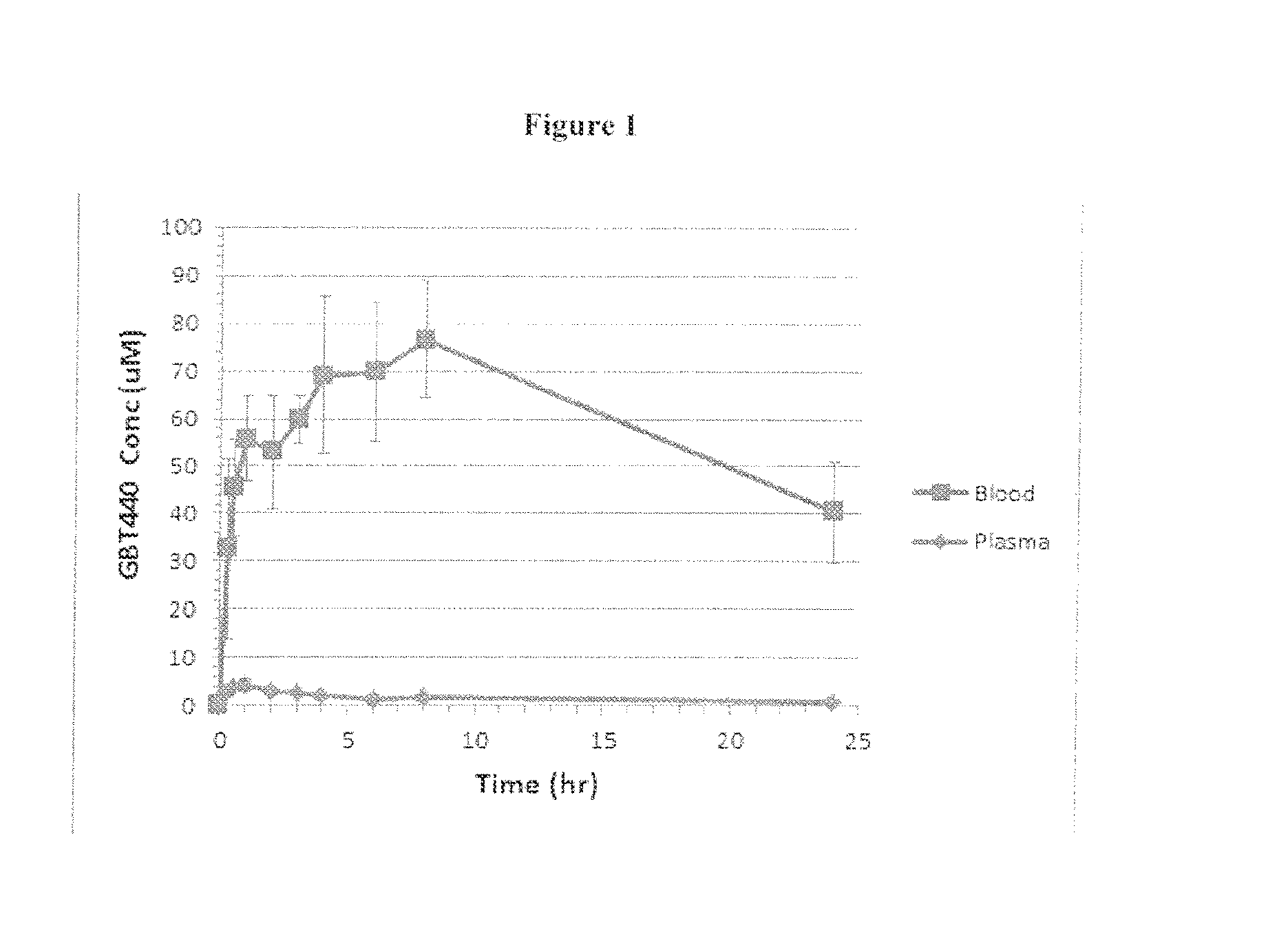
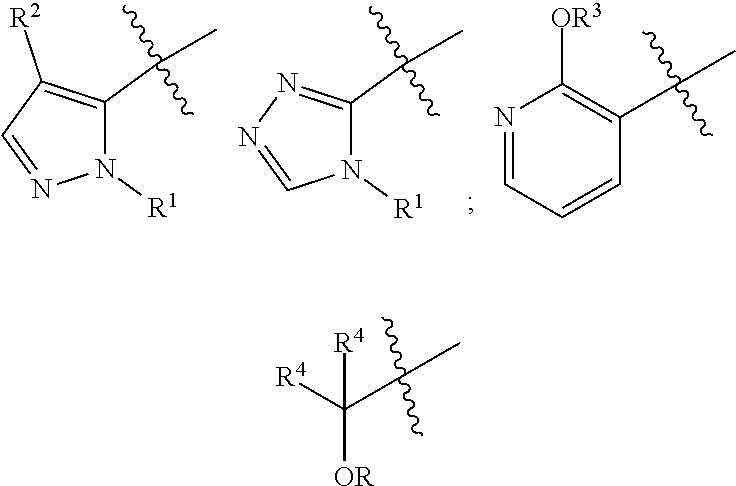
Compounds and uses thereof for the modulation of hemoglobin
a technology of compound and use, applied in the field of compound and use for the modulation of hemoglobin, can solve problems such as blood vessel blockage, and achieve the effect of increasing the oxygen affinity of hemoglobin
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Synthesis of Compound 15
[0235]
[0236]To a solution of 2-bromobenzene-1,3-diol (5 g, 26.45 mmol) in DCM (50 ml) at 0° C. was added DIPFEA ( 11.54 mL, 66.13 mmol) and MOMCl (4.42 ml, 58.19 mmol). The mixture was stirred at 0° C. for 1.5 h, and then warmed to room temperature. The solution was diluted with DCM, washed with sat. NaHCO3, brine, dried and concentrated to give crude product, which was purified by column (hexanes / EtOAc=4:1) to give desired product 15.58 g(90%).
example 2
Synthesis of Compound 13 from 15
[0237]
[0238]To a solution of 2-bromo-1.3-bis(methoxymethoxy)benzene (15) (19.9 g, 71.8 mmol) in THF (150 mL) at −78° C. was added BuLi (2.5 M, 31.6 mL, 79.0 mmol) dropwise. The solution was stirred at −78° C. for 25 min (resulting white cloudy mixture), then it was warmed to 0° C. and stirred for 25 min. The reaction mixture slowly turns homogenous. To the solution was added DMF at 0° C. After 25 min. HPLC showed reaction completed. The mixture was quenched with sat. NH4Cl (150 mL), diluted with ether (300 mL). The organic layer was separated, aq. layer was further extracted with ether (2×200 mL), and organic layer was combined, washed with brine, dried and concentrated to give crude product, which was triturated to give 14.6 g desired product. The filtrate was then concentrated and purified by column to give additional 0.7 g, total mass is 15.3 g.
example 3
Synthesis of Compound 13 from Resorcinol 11
[0239]
[0240]A three-necked round-bottom flask equipped with mechanical stirrer was charged with 0.22 mol of NaH (50 % suspension in mineral oil) under nitrogen atmosphere. NaH was washed with 2 portions (100 mL) of n-hexane and then with 300 mL of dry diethyl ether; then 80 mL of anhydrous DMF was added. Then 0.09 mol of resorcinol 11, dissolved in 100mL of diethyl ether was added dropwise and the mixture was left under stirring at it for 30min. Then 0.18 mol of MOMCl was slowly added. After 1 h under stirring at it, 250 mL of water was added and the organic layer was extracted with diethyl ether, The extracts were washed with brine, dried (Na2SO4)then concentrated to give the crude product that was purified by silica gel chromatography to give compound 12 (93 % yield).
[0241]A three-necked round-bottom flask was charged with 110 mL of n-hexane, 0.79 mol of BuLi and 9.4 mL of tetramethylethylendiamine (TMEDA) under nitrogen atmosphere, the m...
PUM
| Property | Measurement | Unit |
|---|---|---|
| half life | aaaaa | aaaaa |
| pKa | aaaaa | aaaaa |
| pKa | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com