Combination therapy for the treatment of glioblastoma
a glioblastoma and antibody technology, applied in the field of disease and pathological conditions with antivegf antibodies, can solve the problems of high unmet medical needs and limited treatment options of diseases, and achieve the effect of prolonging the patient's median overall survival tim
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
AvaGlio Study
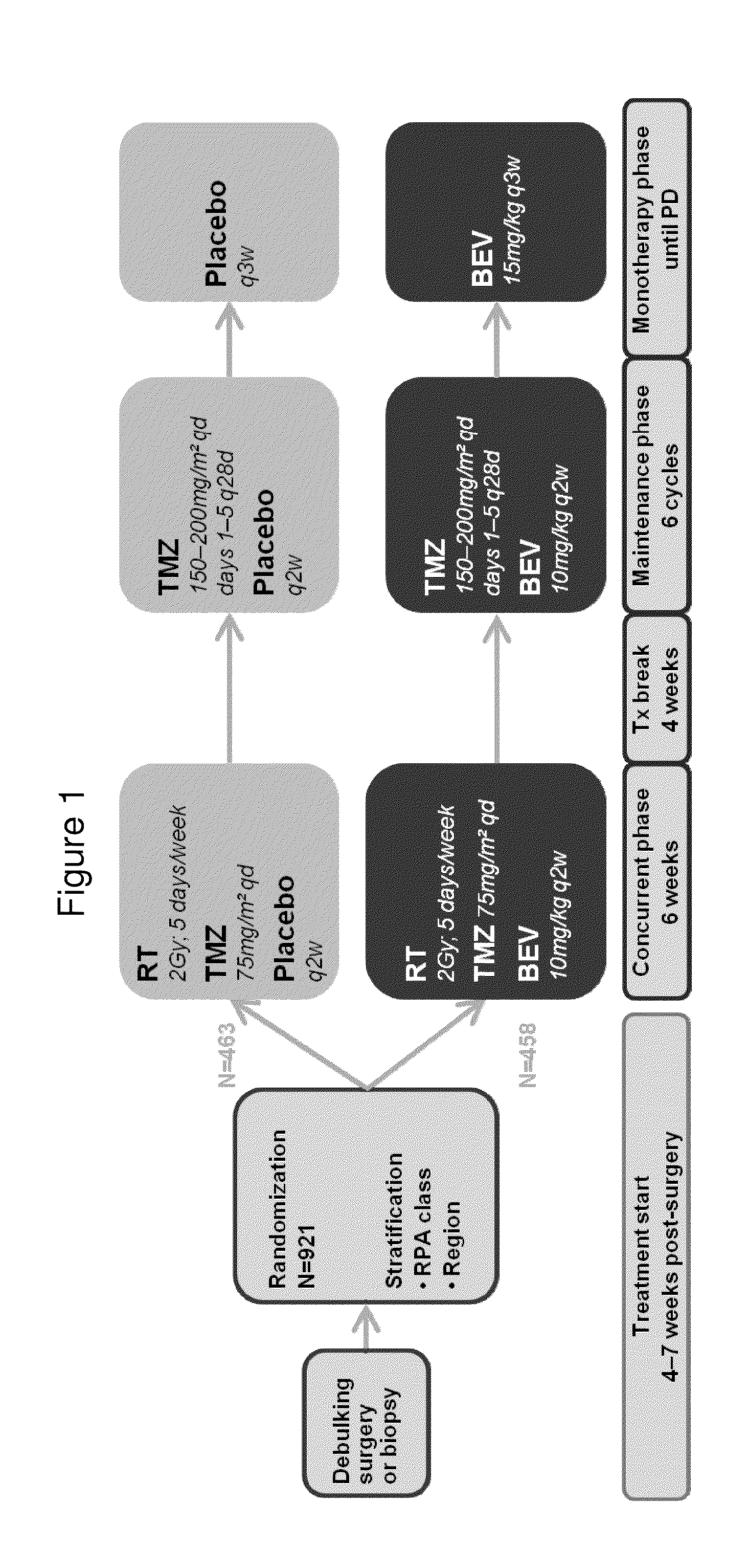
[0146]The AvaGlio trial evaluated the efficacy and safety of bevacizumab in combination temozolomide and radiotherapy for newly diagnosed glioblastoma. This study was designed as a prospective, randomized, double blind, placebo controlled Phase III evaluation of bevacizumab plus chemotherapy versus chemotherapy alone. To be eligible, patients must have had newly diagnosed glioblastoma with a tissue diagnosis that has been established following either a surgical resection or biopsy. By adding bevacizumab to chemotherapy and radiotherapy, the AvaGlio trial aimed to improve overall survival (OS) and progression-free survival (PFS) for this group of patients who had limited therapeutic options and faced a particularly poor prognosis. The primary objective was to compare OS and PFS of patients randomized to temozolomide (TMZ) and radiotherapy only or to temozolomide and radiotherapy plus bevacizumab.
Overview of AvaGlio Study
[0147]This trial consisted of three phases (Concurr...
example 2
Categorizing FFPE-Fixed Samples Using Nanostring Gene Expression Data
[0154]Stratification of clinical samples into gene expression subtypes is complicated by the fact that standard sample preparation, e.g., fixation using formalin-fixed, paraffin-embedded (FFPE) techniques, reduces the quality and amount of RNA available for analysis using standard methods, e.g. microarrays. Recently, single-molecule analysis technologies, e.g. the Nanostring nCounter (Geiss et al. Biotechnol. 26(3): 317-325, 2008), have become available to assay gene expression in FFPE-fixed material.
[0155]To assign patient-derived samples into gene expression subtypes originally defined from fresh-frozen samples analyzed on microarrays, we applied a four-step approach. Specifically, we:[0156]1. Re-normalized published microarray data for 98 samples classified by Lai et al and calculated the gene expression centroids for the 35 classifier genes (reference data).[0157]2. Applied the centroids to classify a new set o...
example 3
Nanostring-Based Classification of Samples from the AvaGlio Trial
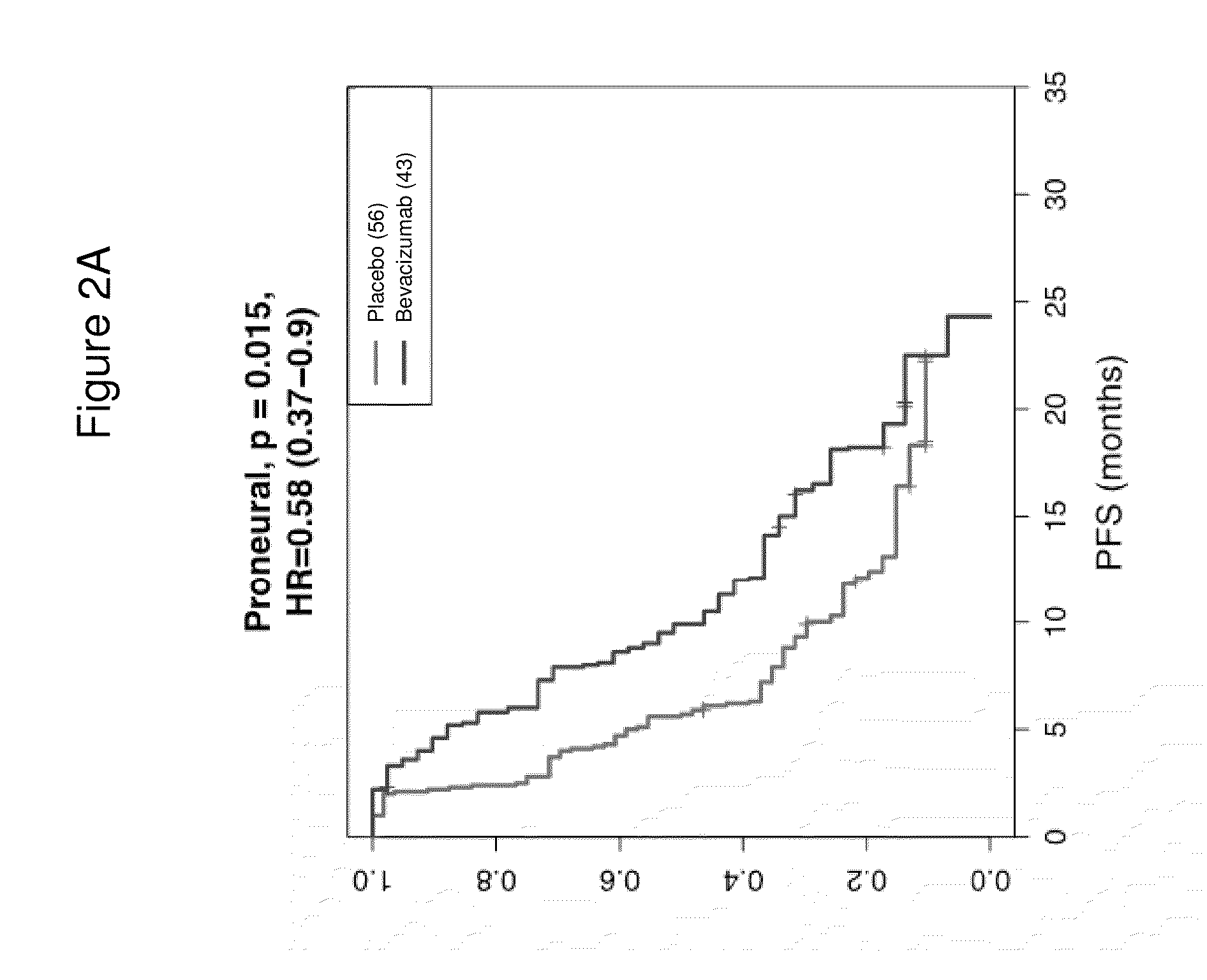
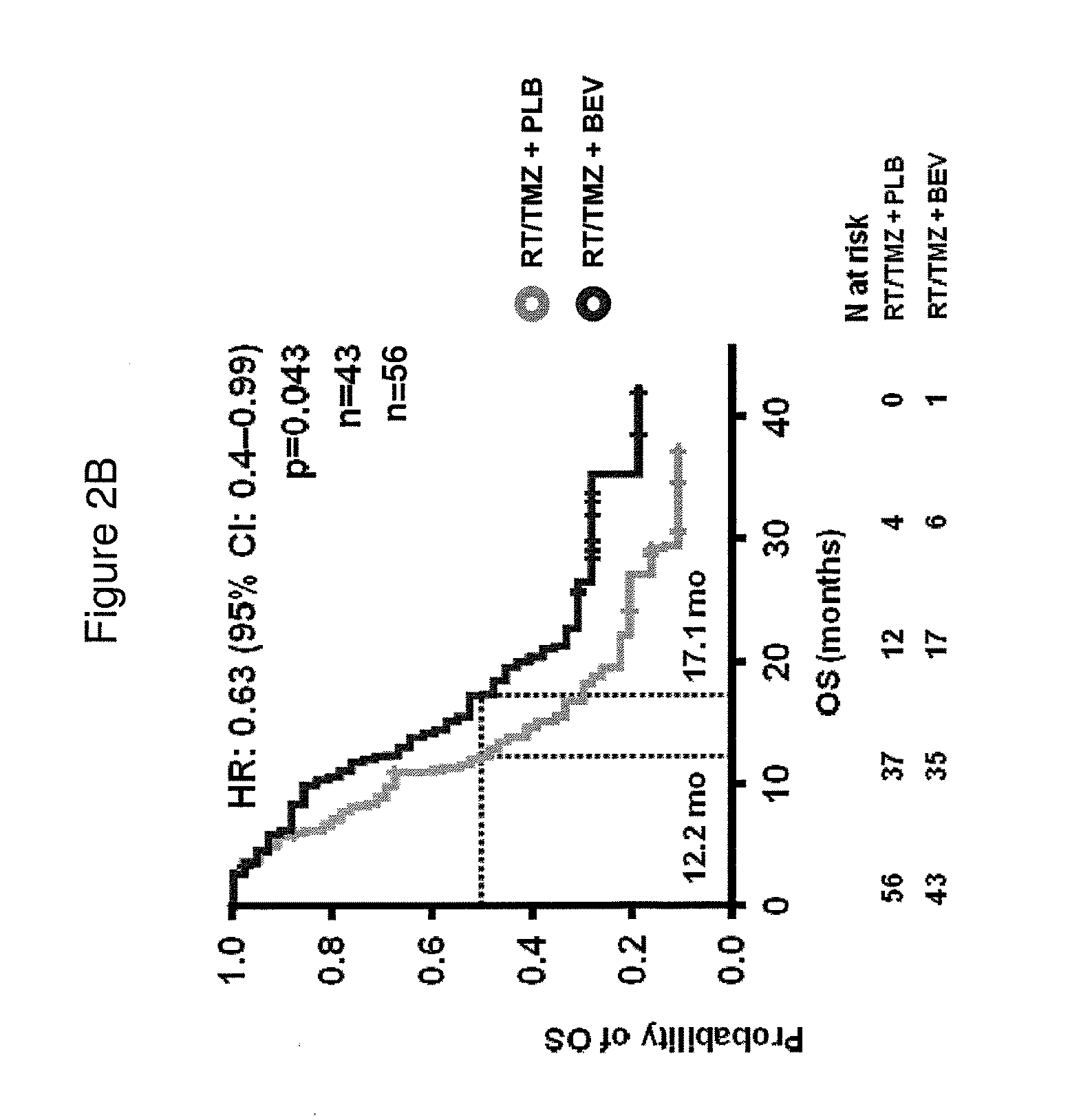
[0171]Next, we classified the 349 samples from the AvaGlio trial for which biomarkers could be evaluated into the gene expression subtypes defined by Phillips (Cancer Cell. 9(3): 157-173, 2006). Selected baseline characteristics of the 349 biomarker evaluable patients within each treatment arm are shown in Table 6.
TABLE 6Selected baseline characteristics of biomarker evaluable patientsBiomarker Evaluable PatientsRT / TMZ / PLBRT / TMZ / BEVPatients, %(n = 178)(n = 171)Median age,58 (21-79)59 (28-84)years (range)GenderMale6460Female3640WHO PS050471-24749RPA class - CRFIII1214IV6359V2224MGMT statusMethylated2623Non-methylated4956Surgical statusBiopsy66Partial resection5244Complete resection3946KPS50-80323290-1006564MMSE score2222≧277573CorticosteroidsOn4740Off4954
Samples were analyzed with the same Nanostring probe set used to derive the subtype-specific centroids and expression scores for the same genes were obtained using the ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Time | aaaaa | aaaaa |
| Time | aaaaa | aaaaa |
| Time | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com