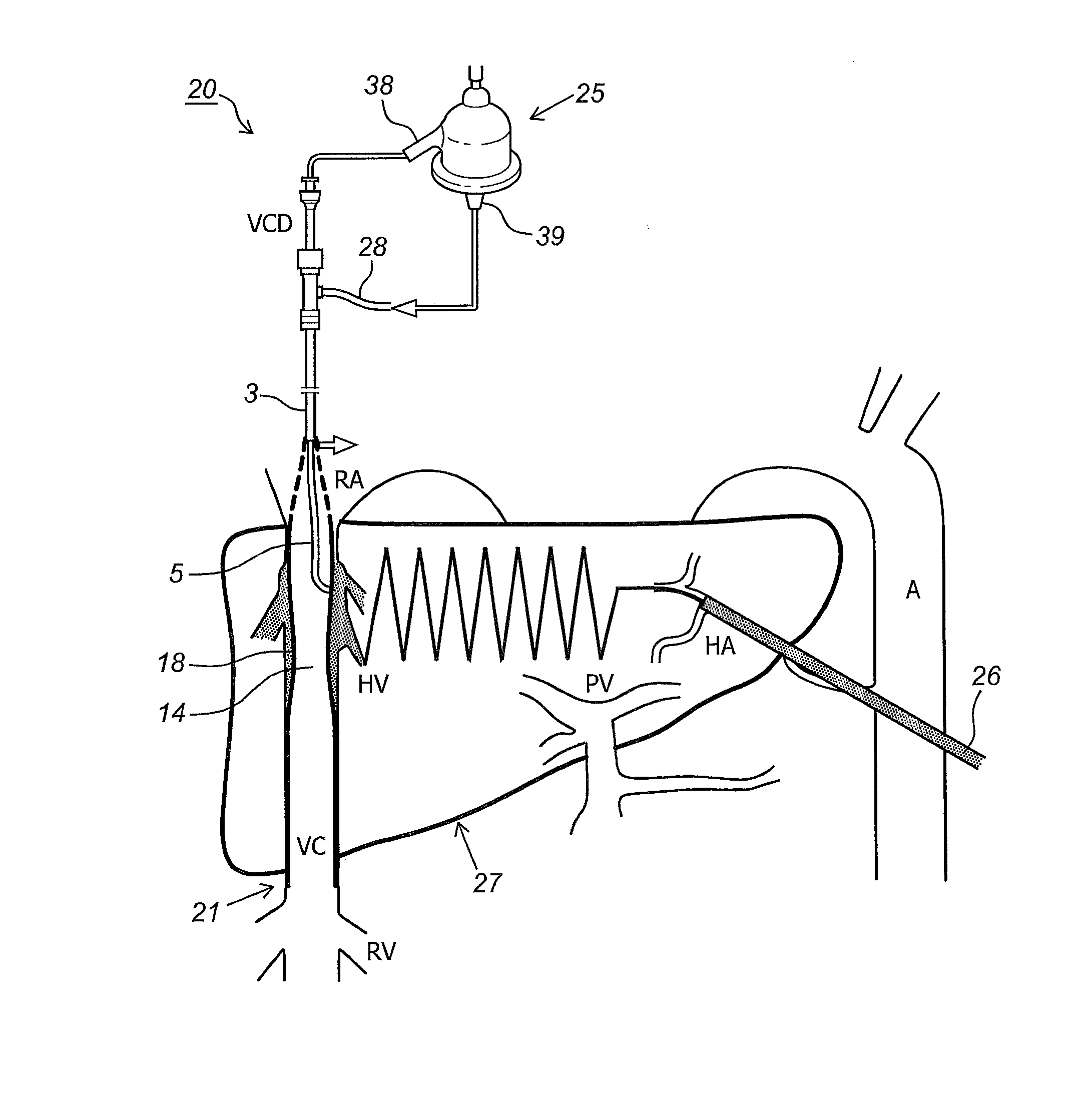
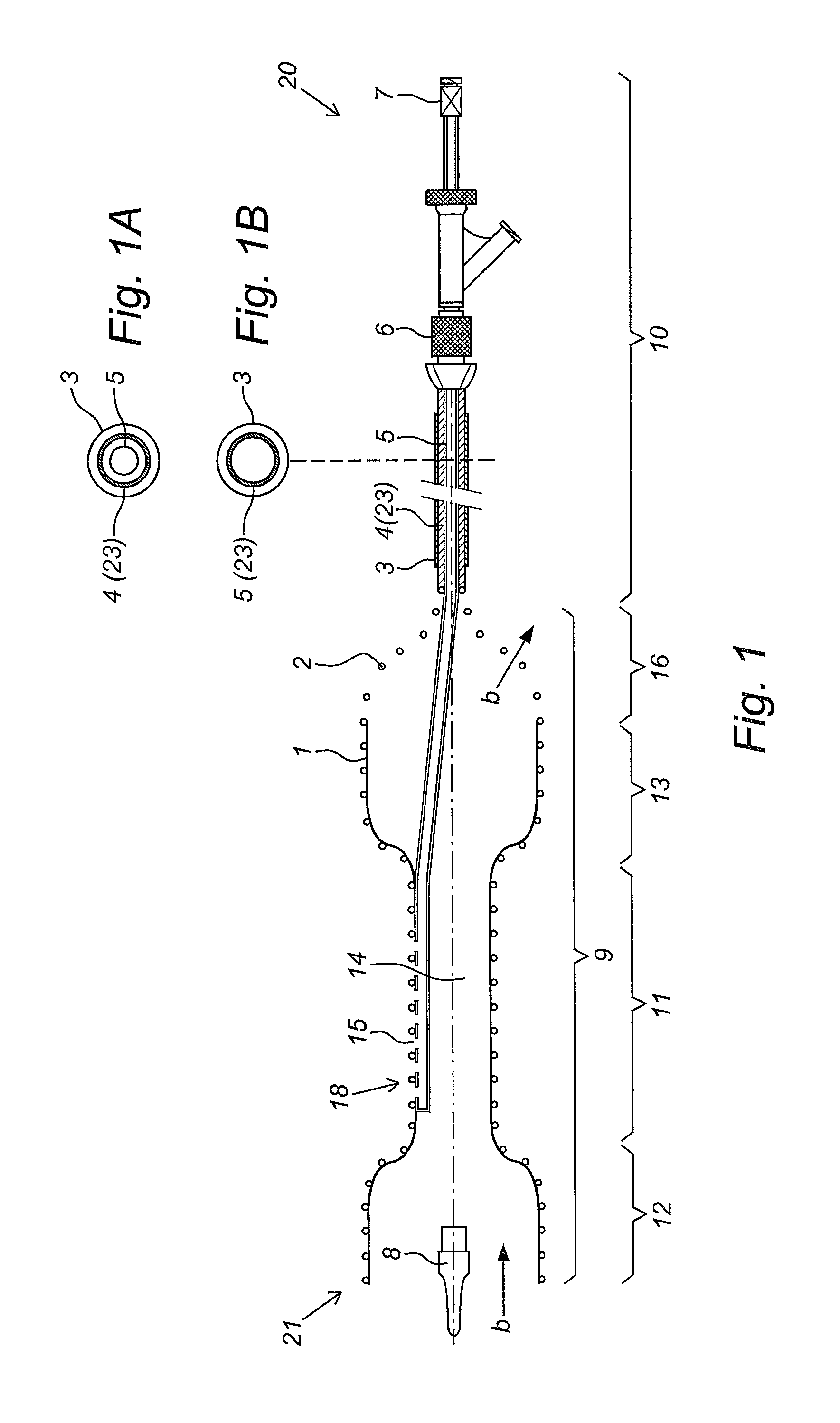
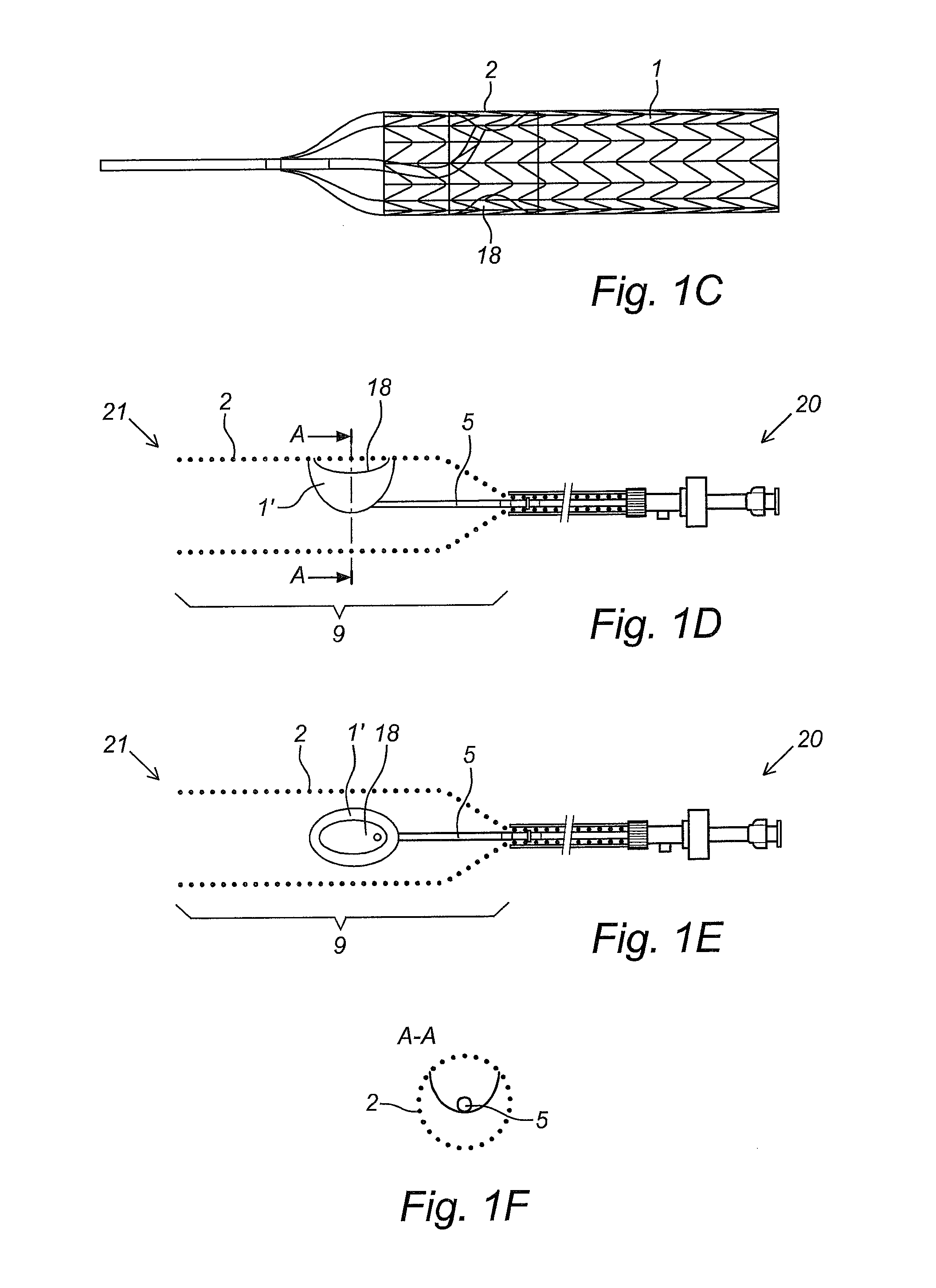
Kit and devices for organ perfusion
a technology for organs and kits, applied in the field of medical treatment systems, can solve the problems of inadvertently reaching other non-targeted organs, affecting the treatment effect, so as to reduce the operative trauma, avoid hospitalization, and shorten the operation procedure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0052]Unless otherwise defined, all terms used in disclosing the invention, including technical and scientific terms, have the meaning as commonly understood by one of ordinary skill in the art to which this invention belongs. By means of further guidance, term definitions are included to better appreciate the teaching of the present invention.
[0053]As used herein, the following terms have the following meanings:
[0054]“A”, “an”, and “the” as used herein refers to both singular and plural referents unless the context clearly dictates otherwise. By way of example, “a compartment” refers to one or more than one compartment.
[0055]“About” as used herein referring to a measurable value such as a parameter, an amount, a temporal duration, and the like, is meant to encompass variations of + / −20% or less, preferably + / −10% or less, more preferably + / −5% or less, even more preferably + / −1% or less, and still more preferably + / −0.1% or less of and from the specified value, in so far such varia...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com