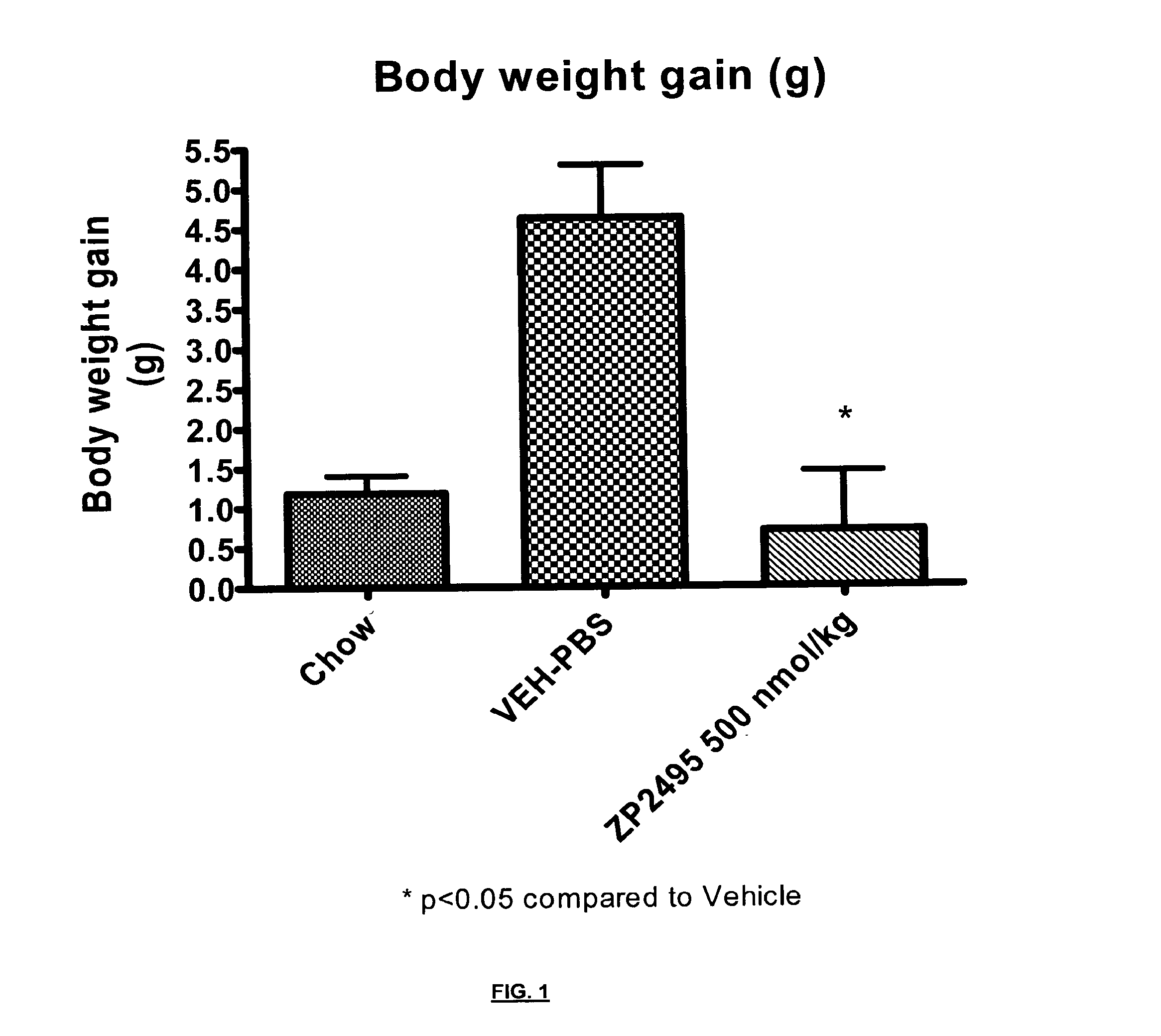
Glucagon analogues
a technology of glucagon and analogues, applied in the field of glucagon analogues, can solve the problems of obesity, reduced life expectancy, and inability of glp-1 to activate the glucagon receptor, so as to prevent weight gain, inhibit or reduce weight gain, and promote weight loss
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Peptide Stability
[0126]
TABLE 2Results for recovery measured by RP-HPLC after stress.ZPRecovery (%)Recovery (%)CompoundSolid peptide0.1M HClGlucagon91152495 (SEQ ID9152NO: 5)2637 (SEQ ID9453NO: 8)2648 (SEQ ID10060NO: 12)2650 (SEQ ID9669NO: 10)2654 (SEQ ID10727NO: 11)
[0127]The results of incubation in HCl stress solutions are shown in Table 2. All compounds as solids were stable over 5 weeks at 40° C. with a recovery of over 90% purity. The results further shows that the tested glucagon analogous have an increased stability towards acidic degradation when compared to native glucagon.
example 2
Efficacy on Glucagon and GLP-1 Receptors
[0128]
TABLE 3 EC50 values of glucagons analogues at Glucagon and GLP-1 receptorsGLP-1RGLUREC50EC50(nM)(nM)Glucagon2.00.10OXM1.00.50Exendin-40.02>1,000ZP2495H-HSQGTFTSDYSKYLDRARADDFVAWLKST-NH20.230.50(SEQ ID NO: 5)ZP2634H-HSQGTFTSDYSKYLDRARADDFVAWLKSA-NH20.200.50(SEQ ID NO: 6)ZP2635H-HSQGTFTSDYSKYLDRARADDFVAWLKEA-NH20.200.20(SEQ ID NO: 7)ZP2637H-HSQGTFTSDYSKYLDRARAEDFVAWLKST-NH20.100.20(SEQ ID NO: 8)ZP2649H-HSQGTFTSDYSKYLDRARADDFVEWLKST-NH20.100.30(SEQ ID NO: 9)ZP2650H-HSQGTFTSDYSKYLDRARADDFVAWLERA-NH20.800.07(SEQ ID NO: 10)ZP2654H-H-DSer-QGTFTSDYSKYLDRARADDFVAWLKST-NH20.800.50(SEQ ID NO: 11)ZP2648H-HSQGTFTSDYSKYLDRARAHDFVAWLKST-NH20.100.20(SEQ ID NO: 12)
example 3
[0129]
TABLE 4Stimulation of lipolysis in primary rat adipocyte cultures(for details see Methods).CompoundEC50 (nM)Exendin-4No effectGlucagon6OXM180ZP2495 (SEQ ID NO: 5)137ZP2648 (SEQ ID NO: 12)26ZP2650 (SEQ ID NO: 10)15ZP2654 (SEQ ID NO: 11)326ZP2649 (SEQ ID NO: 9)57ZP2634 (SEQ ID NO: 6)127ZP2635 (SEQ ID NO: 7)93ZP2637 (SEQ ID NO: 8)42
[0130]The GLP-1 agonist, exendin-4 had no effect on lipolysis in primary adipocyte cultures in contrast to glucagon and OXM (Table 4). ZP2495 (SEQ ID NO: 5) and ZP2634 (SEQ ID NO: 6) were equipotent with OXM, while ZP2648 (SEQ ID NO: 12) and ZP2650 (SEQ ID NO: 10) were 7—12 times as potent as OXM.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com