Methods of developing terpene synthase variants
a technology of terpene synthase and terpene, which is applied in the field of developing terpene synthase variants, can solve the problems of limiting the kinetic capacity required for efficient production of terpenes in engineered microbial hosts, unable to improve the kinetic capacity of terpene synthases, and the application of conventional protein engineering strategies such as directed evolution, which is devoid of terpene synthases. , to achiev
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0200]This example describes methods for making DNA constructs useful in the generation and characterization of terpene synthase variants.
[0201]Expression plasmid pAM36-MevT66 was generated by inserting the MevT66 operon into vector pAM36. Vector pAM36 was generated by removing the tet resistance gene from and adding an oligonucleotide cassette containing AscI-SfiI-AsiSI-XhoI-PacI-FslI-PmeI restriction sites into the pACYC184 vector (GenBank accession number XO6403). The MevT66 operon encoded the set of MEV pathway enzymes that together transform the ubiquitous precursor acetyl-CoA to (R)-mevalonate, namely acetoacetyl-CoA thiolase, HMG-CoA synthase, and HMG-CoA reductase. The MevT66 operon was synthetically generated and comprised the atoB gene of Escherichia coli (GenBank accession number NC—000913 REGION: 2324131 . . . 2325315; encodes an acetoacetyl-CoA thiolase) codon-optimized for expression in Escherichia coli, the coding sequence of the ERG13 gene of Saccharomyces cerevisiae...
example 2
[0234]This example describes methods for making yeast strains useful in the generation and characterization of terpene synthases variants.
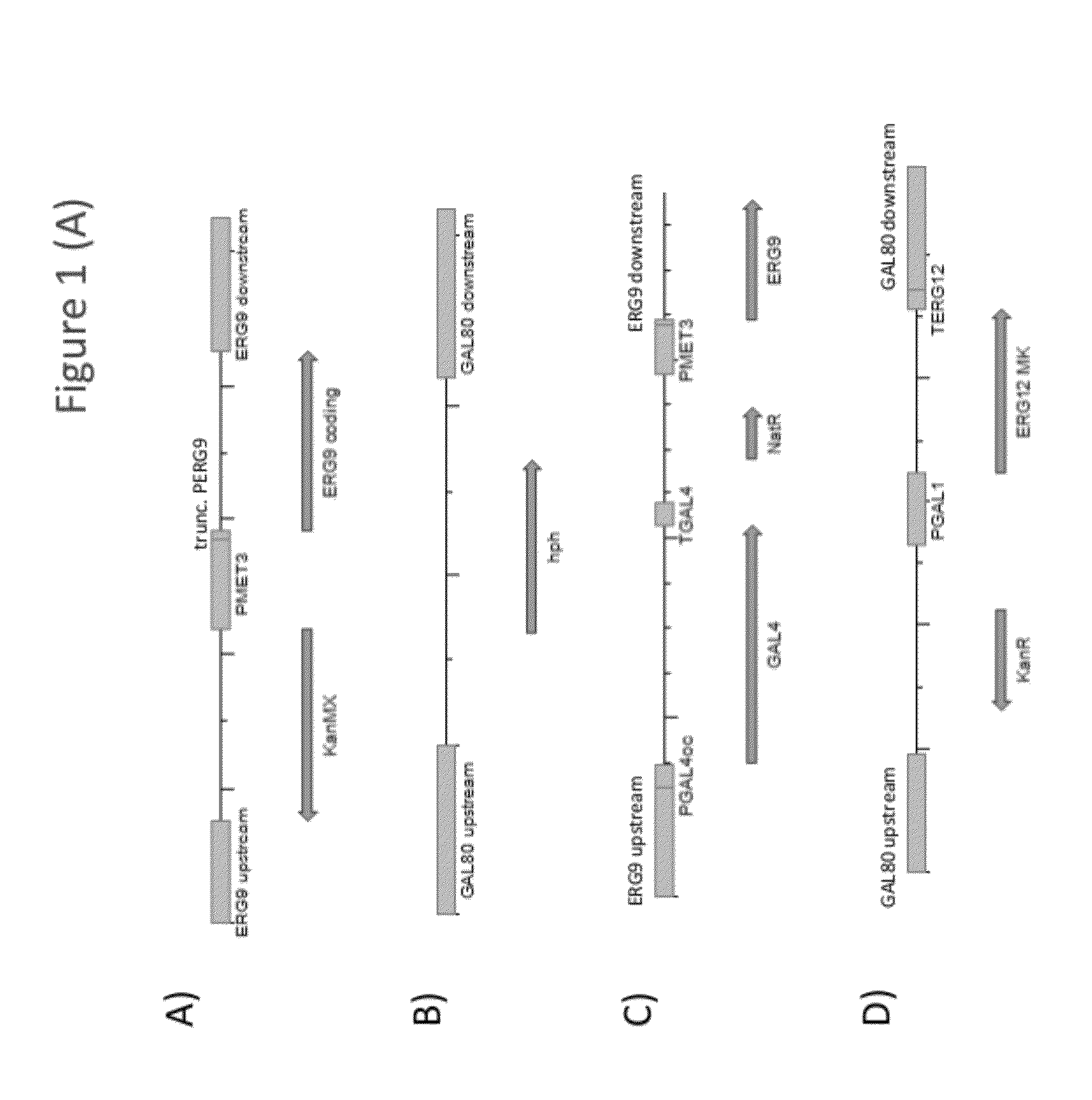
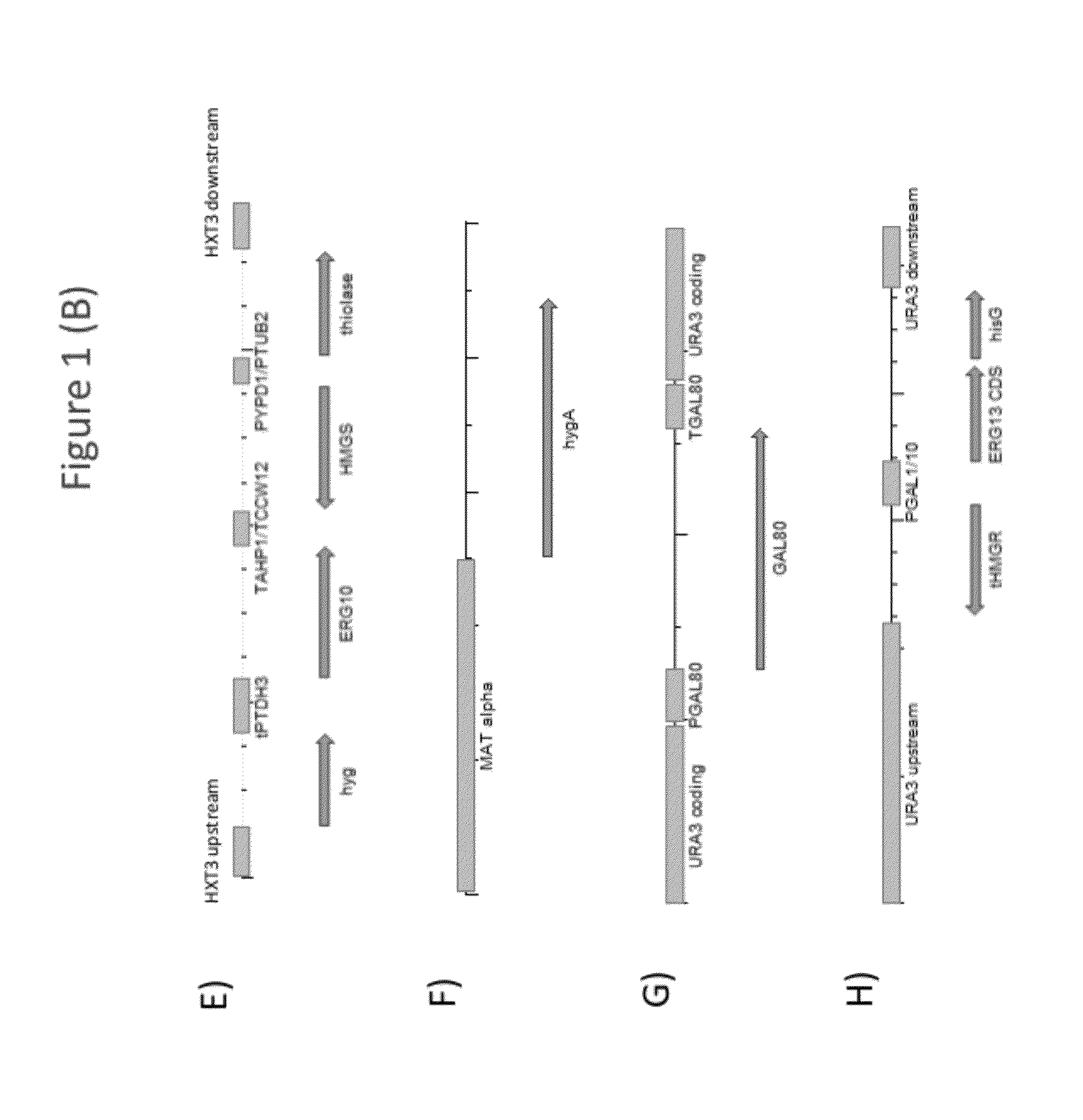
[0235]Strains Y93 (MAT A) and Y94 (MAT alpha) were generated by replacing the promoter of the ERG9 gene of yeast strains Y002 and Y003 (CEN.PK2 background MAT A or MAT alpha, respectively; ura3-52; trp1-289; leu2-3,112; his3Δ1; MAL2-8C; SUC2; van Dijken et al. (2000) Enzyme Microb. Technol. 26:706-714), respectively, with the promoter of the MET3 gene of Saccharomyces cerevisia. To this end, exponentially growing Y002 and Y003 cells were transformed with integration construct i8 (SEQ ID NO: 87), which comprised the kanamycin resistance marker (KanMX) flanked by the promoter and terminator of the Tef1 gene of Kluyveromyces lactis, the ERG9 coding sequence, a truncated segment of the ERG9 promoter (trune. PERG9), and the MET3 promoter (PMET3), flanked by ERG9 upstream and downstream sequences (FIG. 1A). Host cell transformants were selected on mediu...
example 3
[0283]This example demonstrates the feasibility of using FPP starvation based selection in Escherichia coli to screen for terpene synthases with improved in vivo performance.
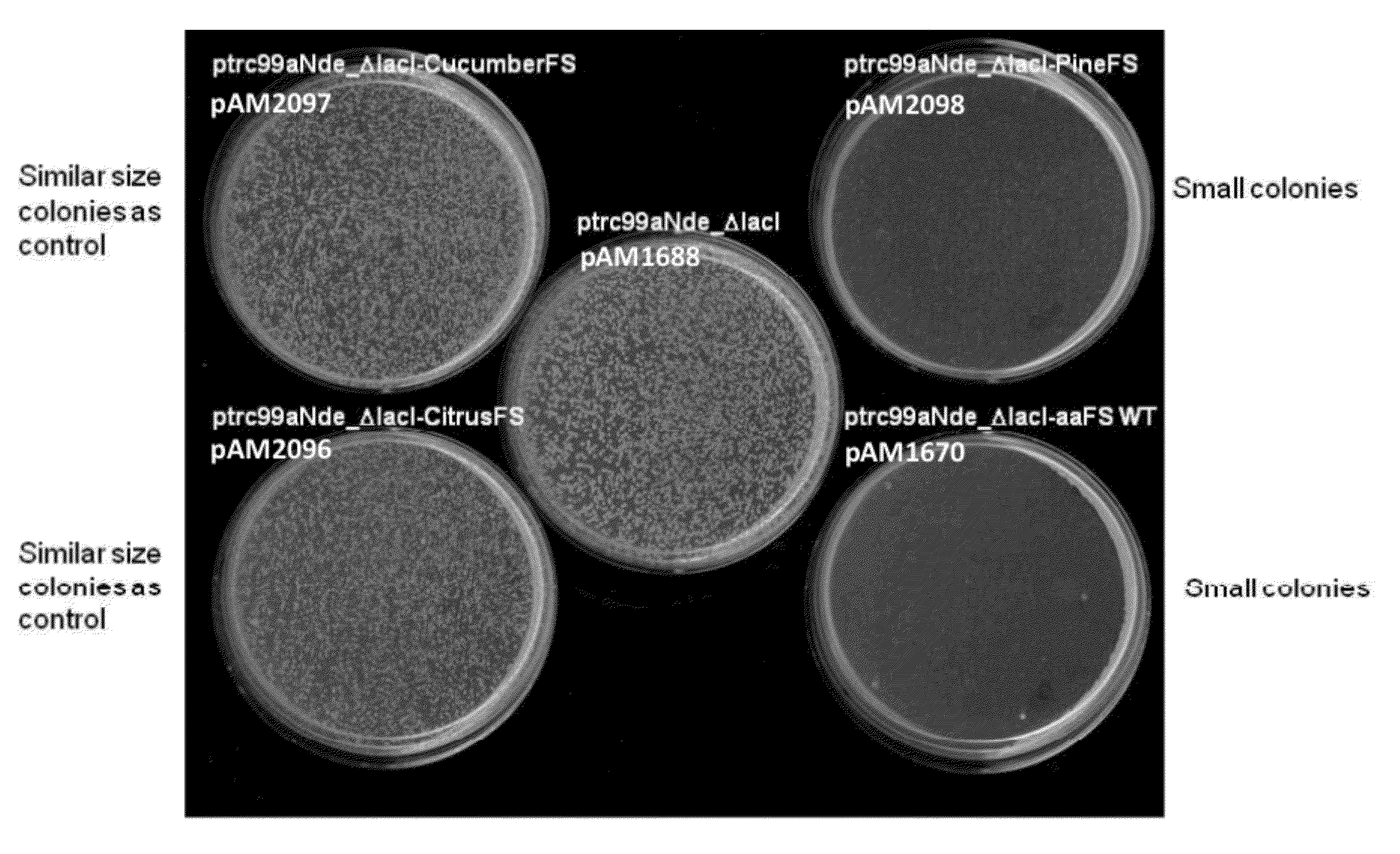
[0284]DH5αchemical- or electro-competent Escherichia coli cells (Invitrogen, Carlsbad, Calif.) were transformed with 5 ng of an expression plasmid selected from the group consisting of expression plasmids pAM1668 (negative control), pAM1670, pAM2096, pAM2097, pAM2098, pAM2101, and pAM2104. Host cell transformants were plated on agar plates comprising carbenicillin, and the plates were incubated at 30° C. for 2-3 days.
[0285]As shown in FIG. 2, cells transformed with expression plasmid pAM2097 or pAM2096 produced colonies of similar size as cells transformed with empty vector (pAM1668). However, cells transformed with expression plasmid pAM1670, pAM2098, as well as cells transformed with expression plasmids pAM2104 or pAM2101 (data not shown), produced colonies that were smaller than those produced by the control....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com