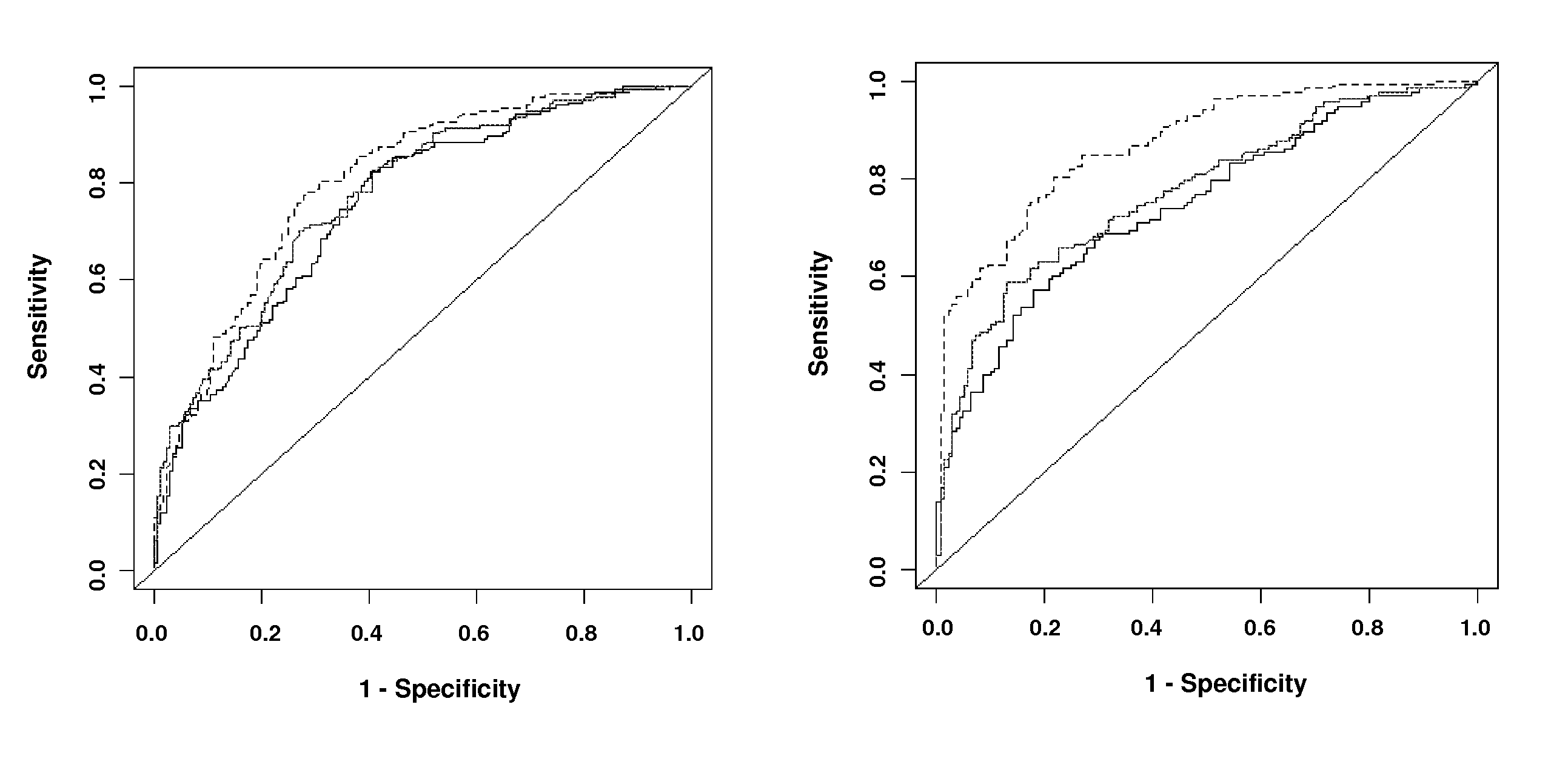
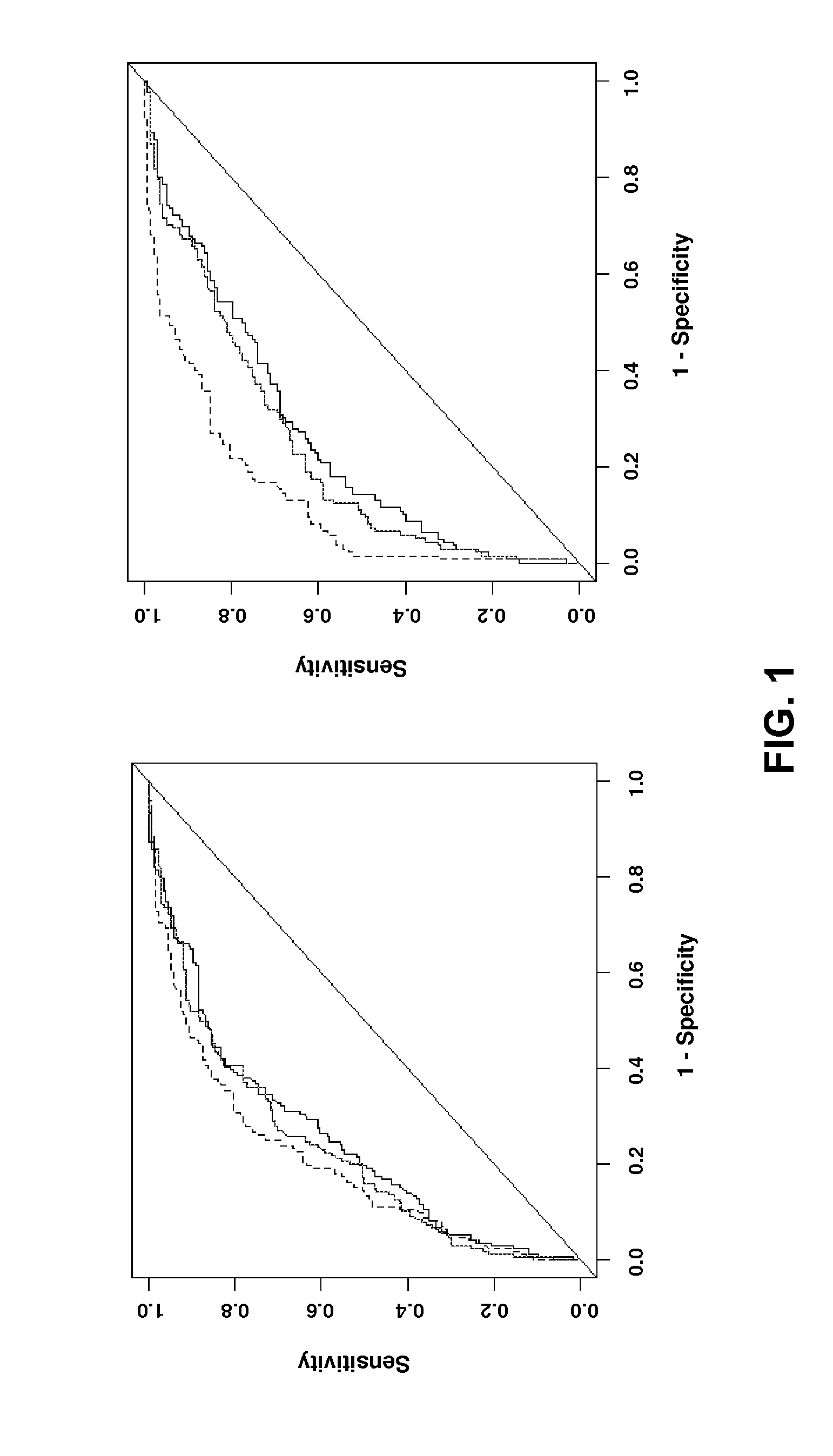
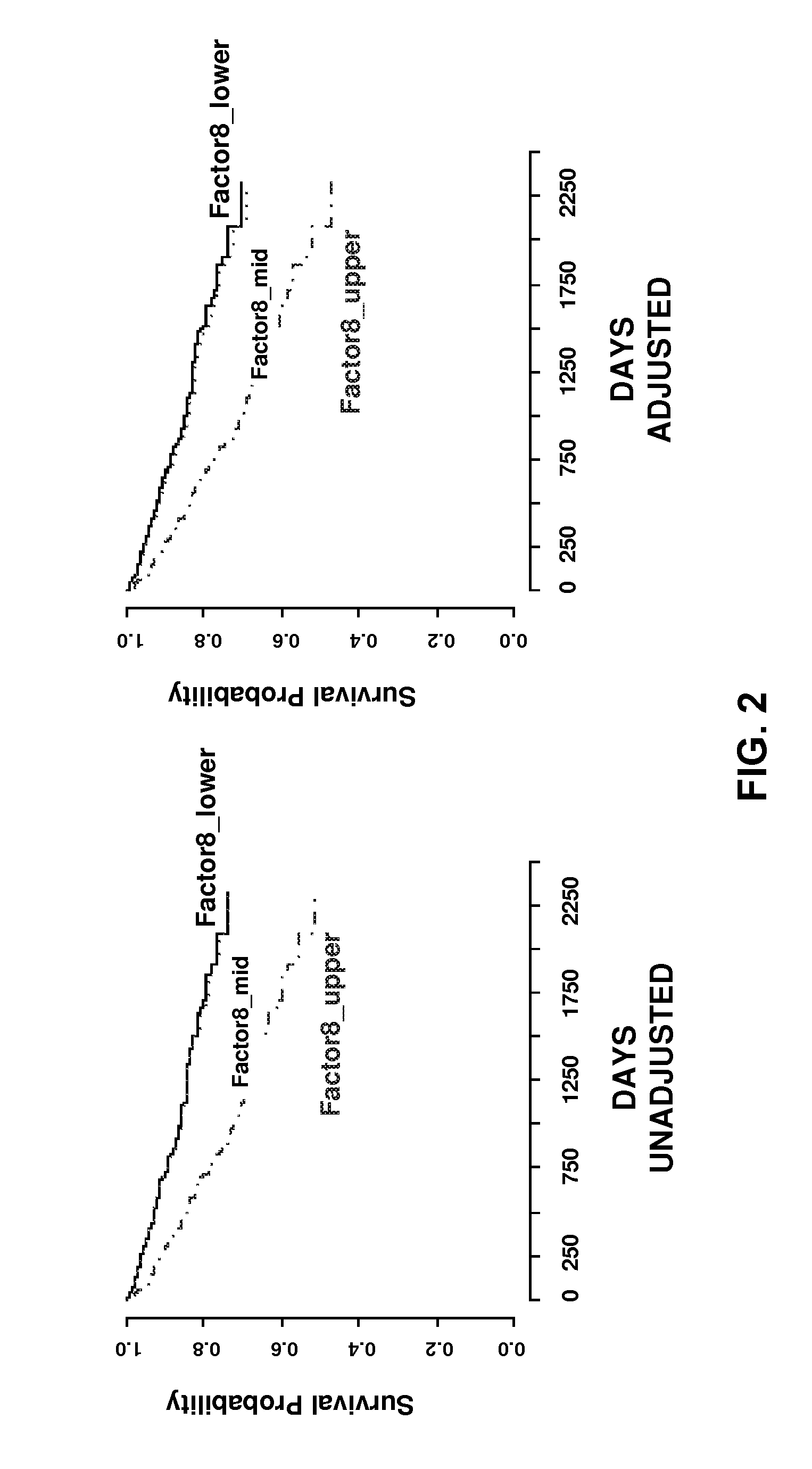
Predicting coronary artery disease and risk of cardiovascular events
a technology of coronary artery disease and risk, applied in the direction of instruments, separation processes, applications, etc., can solve the problem of incomplete mechanistic understanding of cad risk
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Association of Metabolites with CAD and Risk of Cardiovascular Events Methods
Study Sample
[0033]The CATHGEN biorepository consists of subjects recruited sequentially through the cardiac catheterization laboratories at Duke University Medical Center (Durham, N.C.). After informed consent, blood was obtained from the femoral artery at time of arterial access for catheterization, immediately processed to separate plasma, and frozen at −80° C. All subjects were fasting for a minimum of six hours prior to collection. Clinical data were provided by the DDCD, a database of patients undergoing cardiac catheterization at Duke University since 1969. Medication data were collected for medications used chronically, i.e. medications at admission (inpatients) or from a clinic note within one month prior (outpatients). Follow-up data, including occurrence of myocardial infarction (MI) and death were collected at six months after catheterization, then annually thereafter. Vital status was confirmed ...
example 2
Heritability of CAD
Materials and Methods
[0061]Study Population. The GENECARD study enrolled 920 families to perform affected-sibling-pair linkage for identification of genes for early-onset CAD (before age 51 for men, age 56 for women) (Hauser et al., 2003, Am Heart J, 145, 602-613). Families with at least two siblings each of whom met the criteria for early-onset CAD (before age 51 for men, age 56 for women) were recruited. Unaffected family members were defined as no clinical evidence of CAD and age greater than 55 years for men (greater than 60 years for women). From this cohort, we selected eight representative families we believed would be particularly informative, based on availability of a relatively large number of family members and a heavy burden of CAD in the proband and surrounding generations (FIG. 3). These families were recontacted; the affected-sibling-pair and family members not previously enrolled were ascertained regardless of CAD, focused on offspring of the affe...
PUM
| Property | Measurement | Unit |
|---|---|---|
| mass spectroscopy | aaaaa | aaaaa |
| colorimetric | aaaaa | aaaaa |
| mass spectrometry | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com