Cerebrospinal fluid drainage
a cerebrospinal fluid and percutaneous adjustment technology, applied in the direction of wound drains, intravenous devices, medical devices, etc., can solve the problems of uncontrollable discharge, increased risk of infection in external drainage, and no longer justified external drainage after seven to ten days, so as to achieve the effect of reducing the weight of the closing element and facilitating design freedom
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
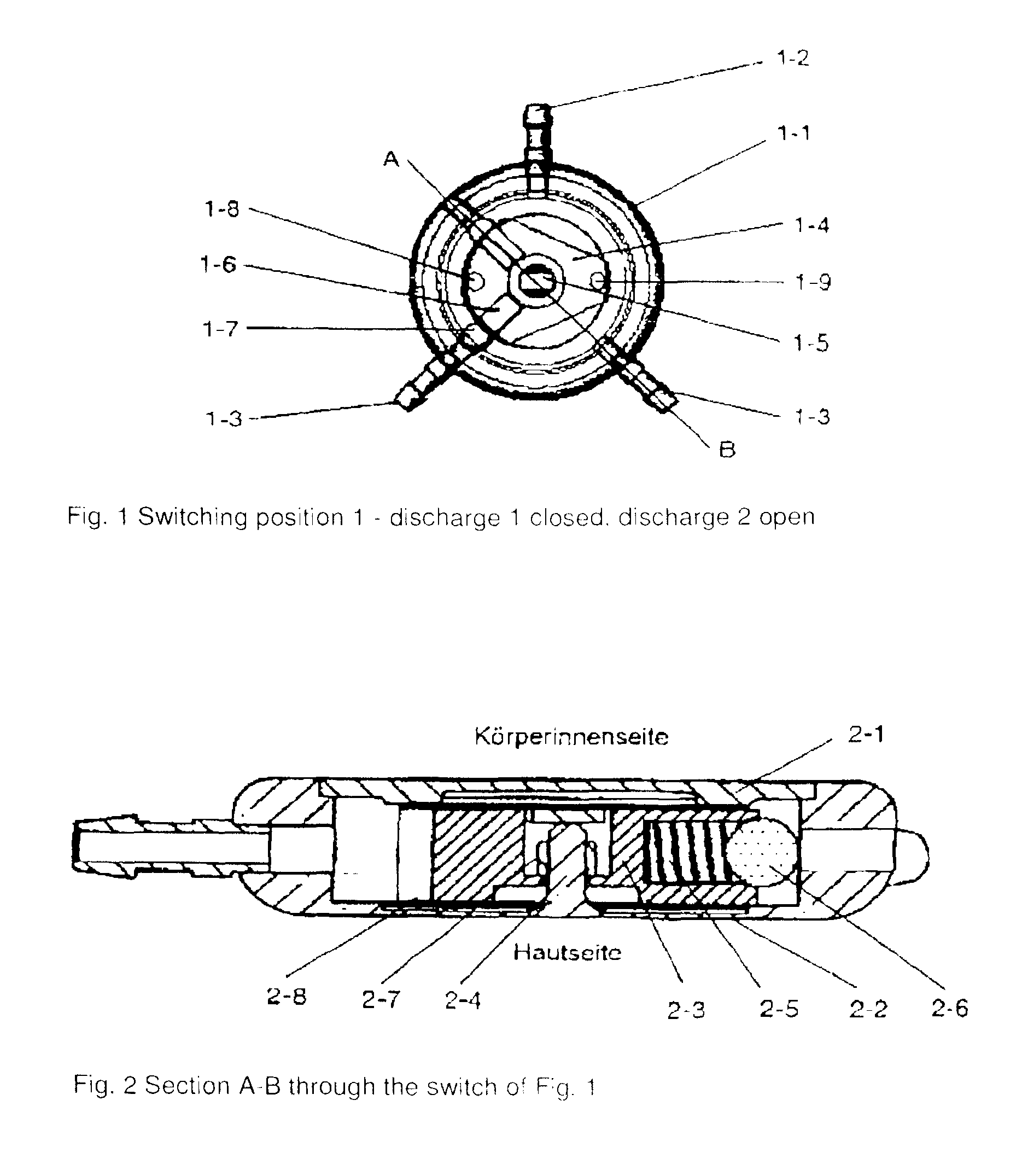
[0078]In the drawing of FIG. 1 an embodiment of the present application is illustrated in the switch position 1 (discharge 1 closed, discharge 2 open).
[0079]In the drawing of FIG. 2 is illustrated the view of the section A-B of the switch of FIG. 1.
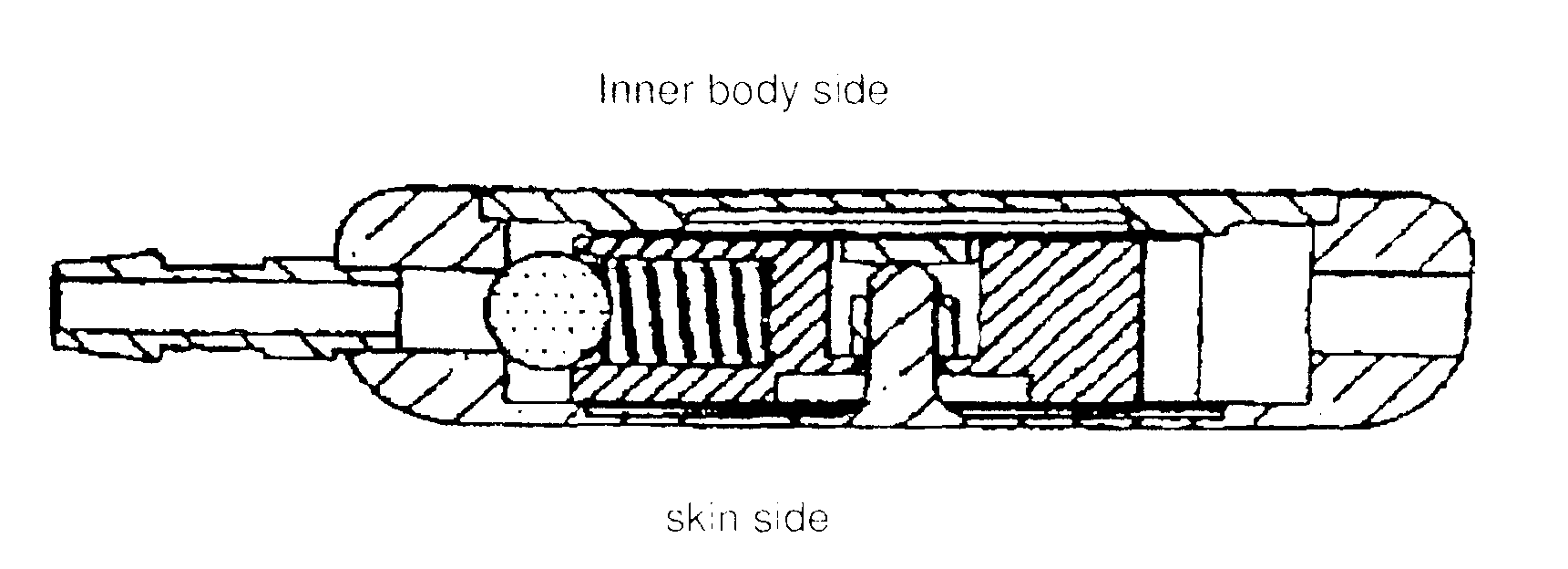
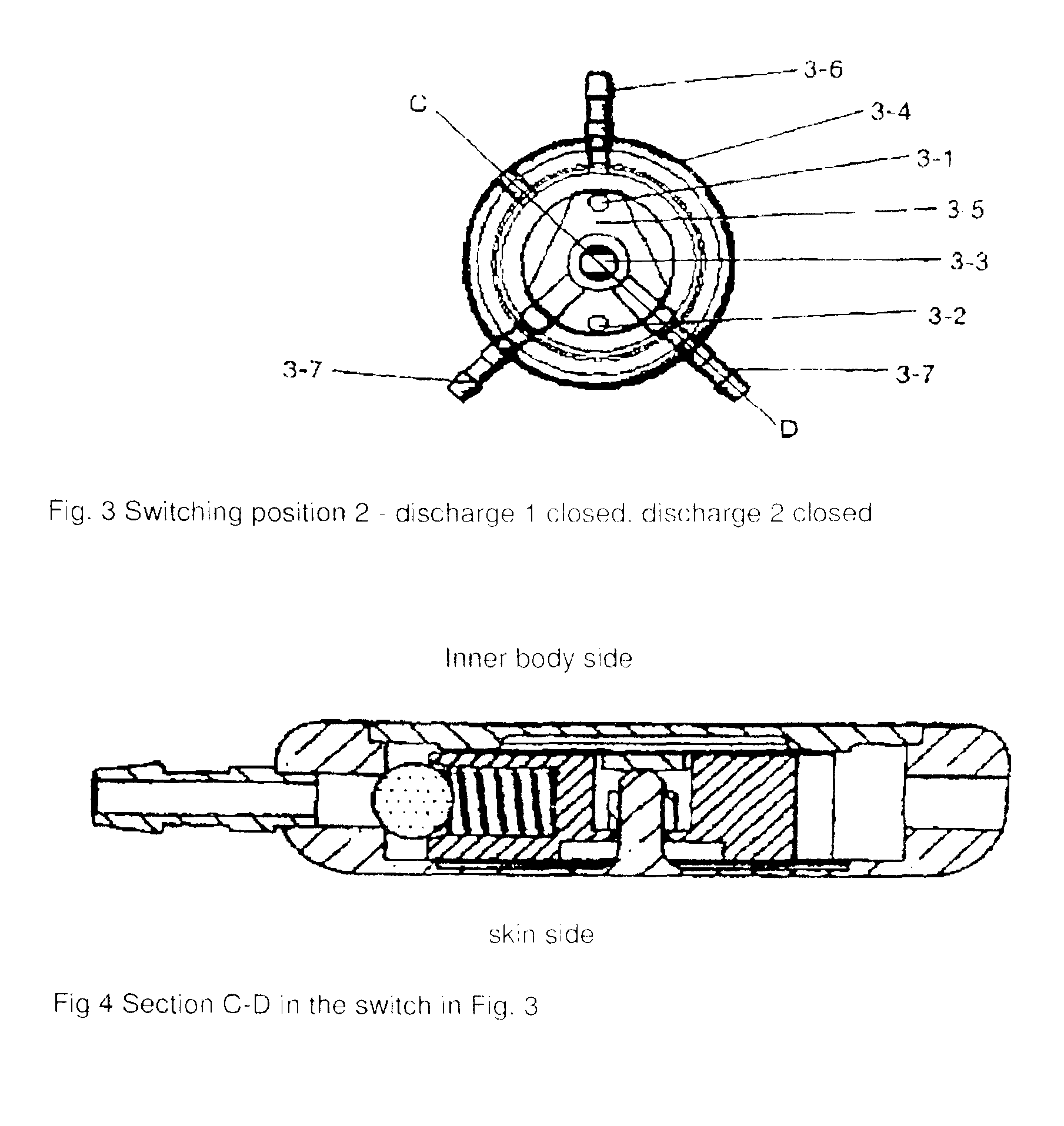
[0080]The two-way discharge switch comprises a solid metallic housing 1-1, a lid 1-2, a base 2-2 as well as an inlet port 1-2 and outlet port 1-3. Moreover, in the embodiment, the 2-way discharge switch possesses a closing element 1-4 / 2-3 that is mounted on the shaft 1-5 / 2-4, a spring system 1-6 / 2-5 with a ball 1-7 / 2-6 for the precision non-return valve and a locking device 2-7. The two-way discharge switch is located in a tube that is implanted in a patient for cerebrospinal fluid drainage. The two-way discharge switch has three switching states. Switching state 1 closes discharge 1 and opens discharge 2, switching state 2 closes both discharges 1 and 2, and switching state 3 closes discharge 2 and opens discharge 1. In the switching sta...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com