Au-Ga-In Brazing Material
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
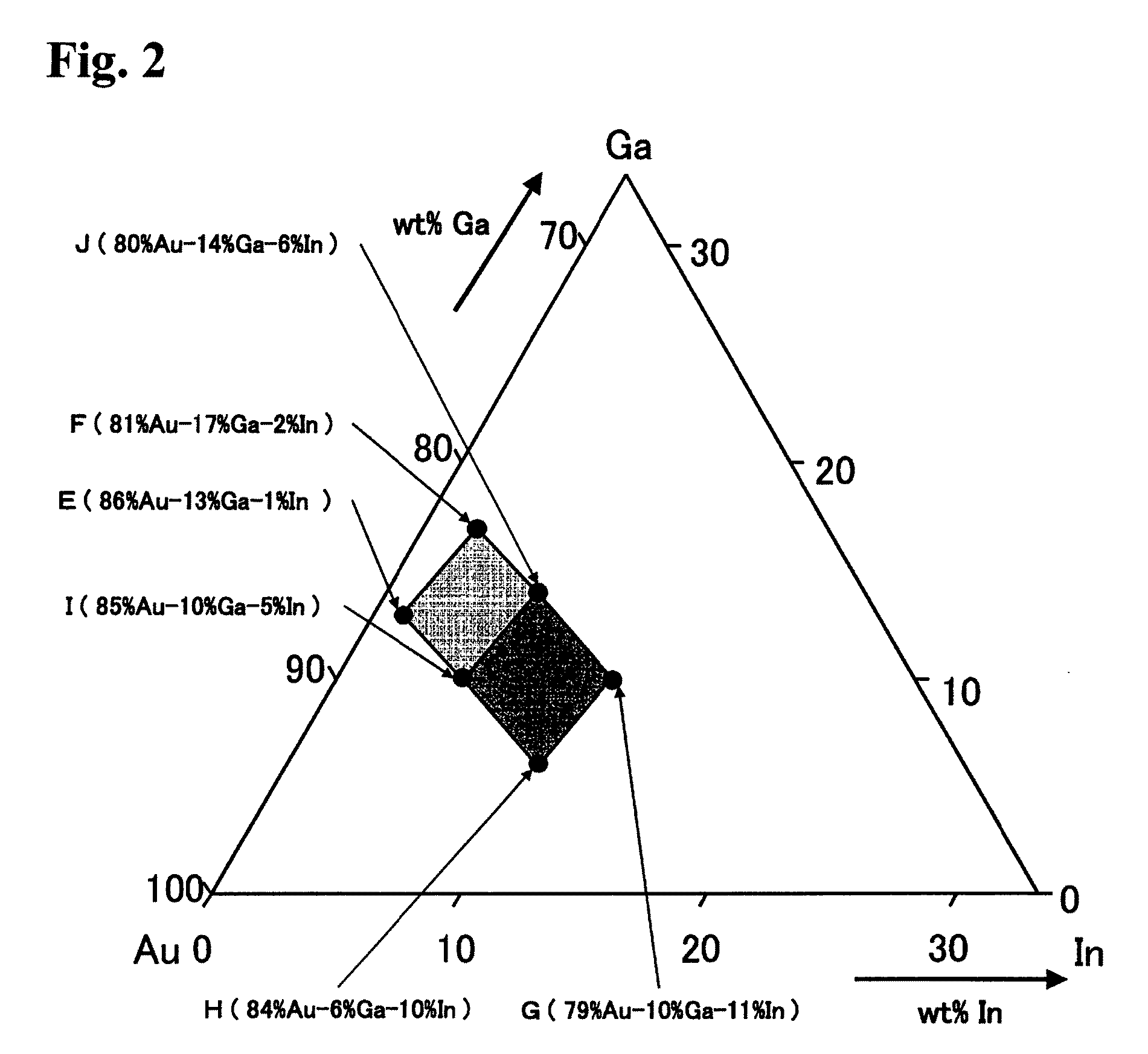
[0028]Hereinafter, an embodiment of the present invention and comparative example will be described. In this embodiment, a brazing material made of a Au—Ga—In alloy having various compositions lying within and out of a region of FIG. 1, and a brazing material in which Sn was added to a Au—Ga—In alloy were manufactured. The properties of the brazing materials were considered. In manufacture of samples, metals weighed so as to have a prescribed composition were melted, cast, and subjected to rolling to produce brazing materials having a thickness of 50 μm.
[0029]The manufactured brazing materials were first evaluated for hardness, processability and melting properties (liquidus, solidus). The brazing materials were evaluated for the hardness with a Vickers hardness meter. The processed brazing materials were evaluated for the processability by observing the existence or nonexistence of occurrence of break and crack of the brazing materials with a stereoscopic microscope (10 times). The...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com