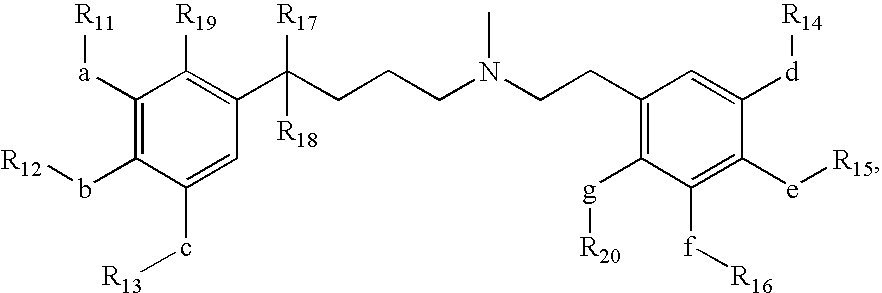
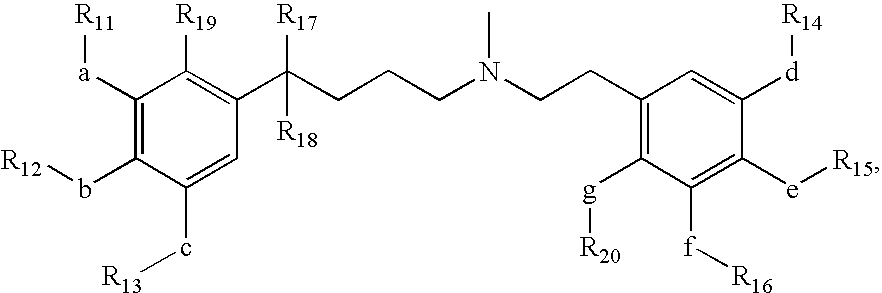
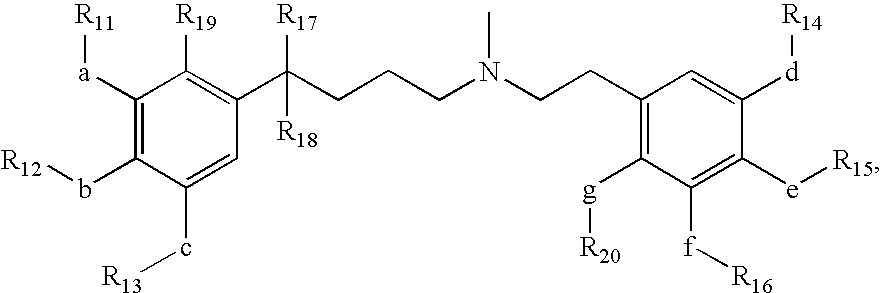
Short acting phenylalkylamine calcium channel blockers and uses thereof
a technology of phenylalkylamine and calcium channel blocker, which is applied in the field of short-acting phenylalkylamine calcium channel blocker, can solve the problems of insufficient blood oxygen supply, permanent damage to the heart, and irregular heartbea
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
5-Bromo-2-(3,4-dimethoxyphenyl)-2-isopropylpentanenitrile (1f)
[0321]Method A Step 1: To a solution of 9.99 g (56.4 mmol) of (3,4-Dimethoxyphenyl)acetonitrile in 141 mL of tetrahydrofuran (THF) at −30° C., was slowly added 56.4 mL (56.4 mmol) of sodium bis(trimethylsilyl)amide (NaHMDS, 1.0 M in THF). The mixture was stirred at −30° C. for 10 minutes and 10.6 mL (113.0 mmol) of 2-bromopropane was added. The mixture was heated to reflux for 2 hours (h) then left at 22° C. for about 16 h. A saturated aqueous solution of NH4Cl was added and the mixture was extracted with ethyl acetate. The organic layer was washed with brine, dried (Na2SO4), filtered and evaporated. The residue was purified by flash chromatography on silica gel eluting first with hexane and then gradually increasing to 15% ethyl acetate / hexane to give 2-(3,4-dimethoxyphenyl)-3-methylbutanenitrile as an oil.
[0322]Method A Step 2: To a solution of 11.21 g (51.1 mmol) of 2-(3,4-dimethoxyphenyl)-3-methylbutanenitrile in 126 ...
example 2
1a: Dimethyl 2-(3-bromopropyl)-2-(3,4-dimethoxyphenyl)malonate
[0324]For Step 1, (3,4-dimethoxy-phenyl)-acetic acid methyl ester was substituted for (3,4-Dimethoxyphenyl)acetonitrile, dimethyl carbonate was substituted for 2-bromopropane and sodium hydride was substituted for NaHMDS. For Step 2, sodium hydride was substituted for NaHMDS.
example 3
1b: Methyl 5-bromo-2-cyano-2-(3,4-dimethoxyphenyl)pentanoate
[0325]For Step 1, dimethyl carbonate was substituted for 2-bromopropane and sodium hydride was substituted for NaHMDS.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com