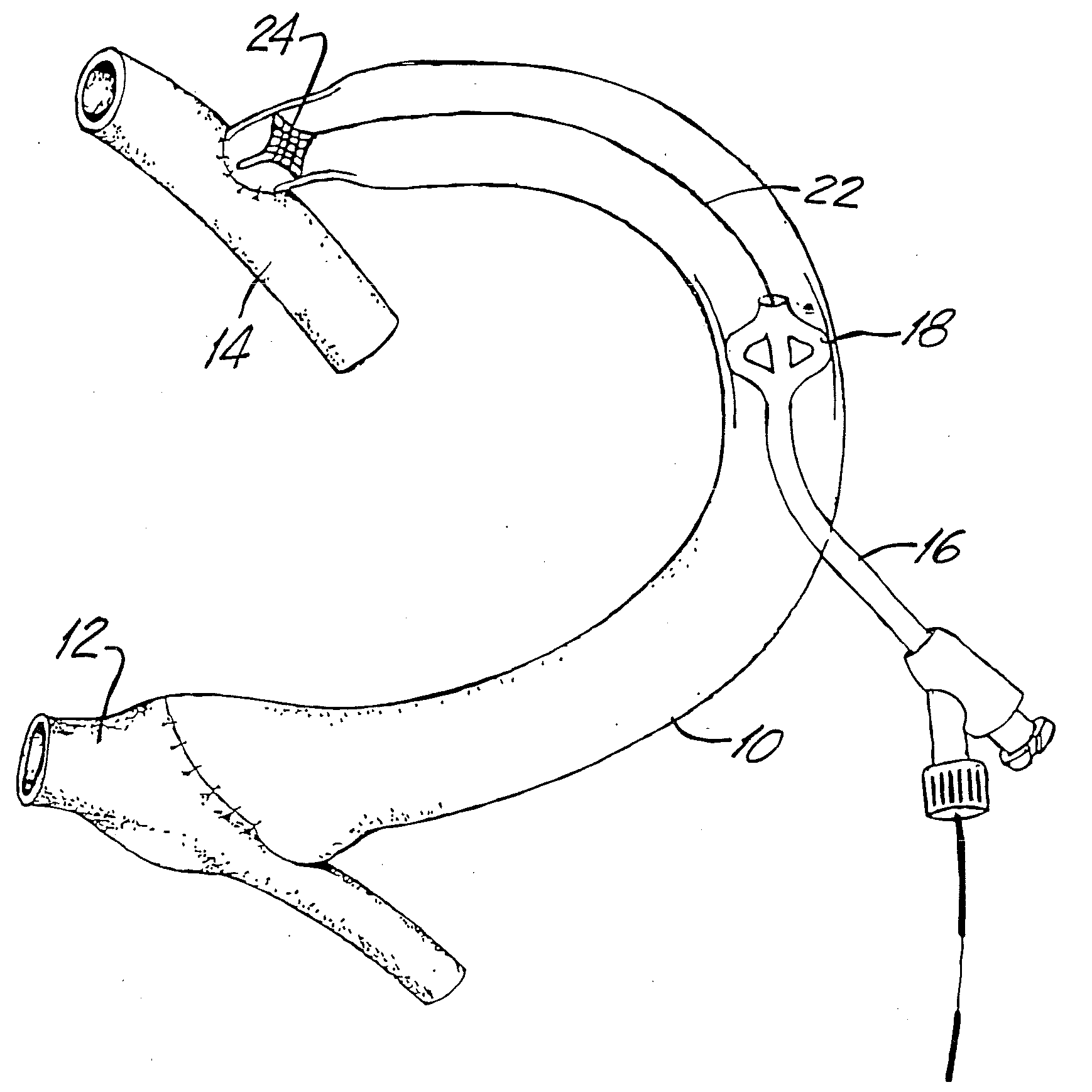
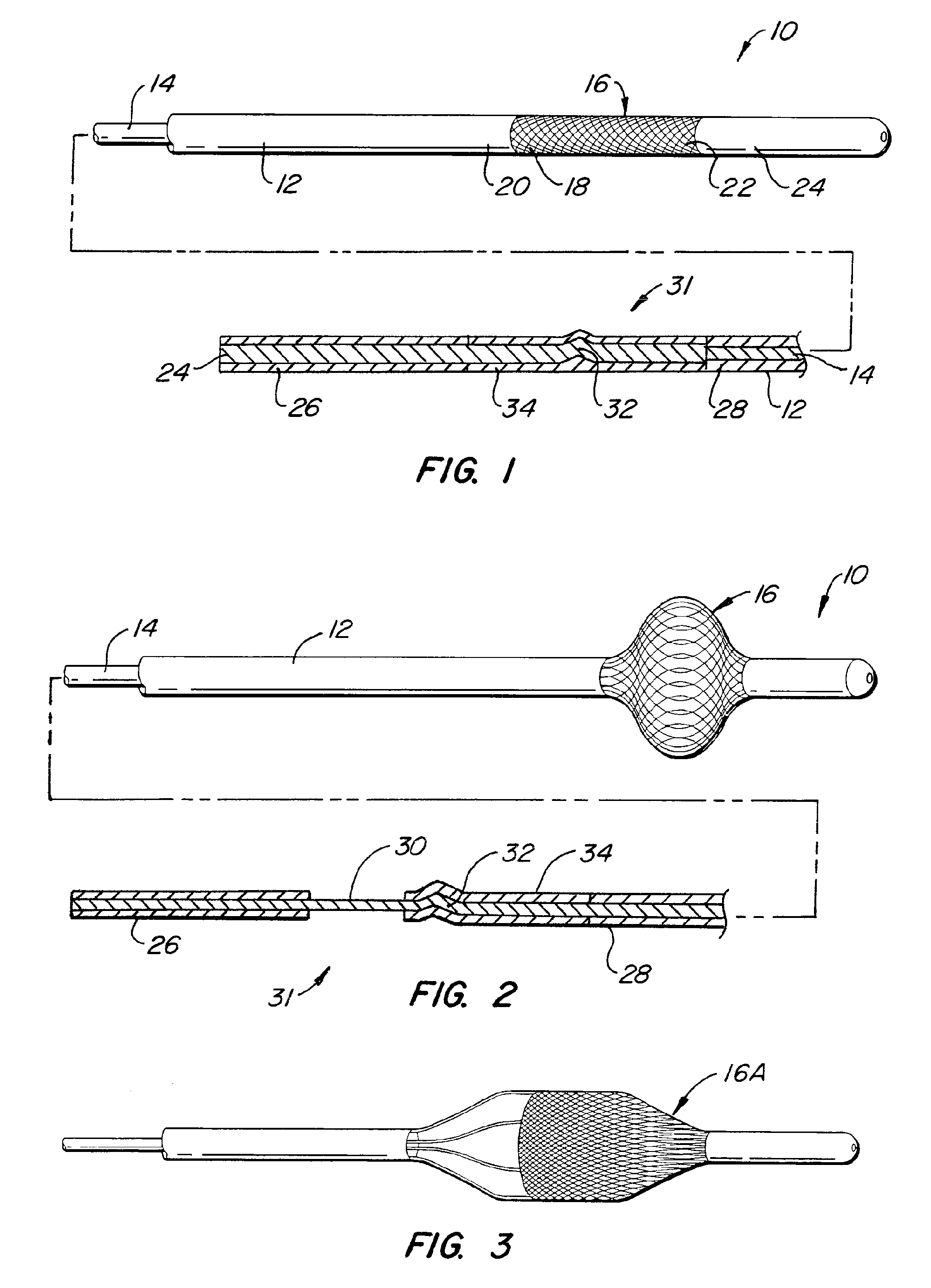
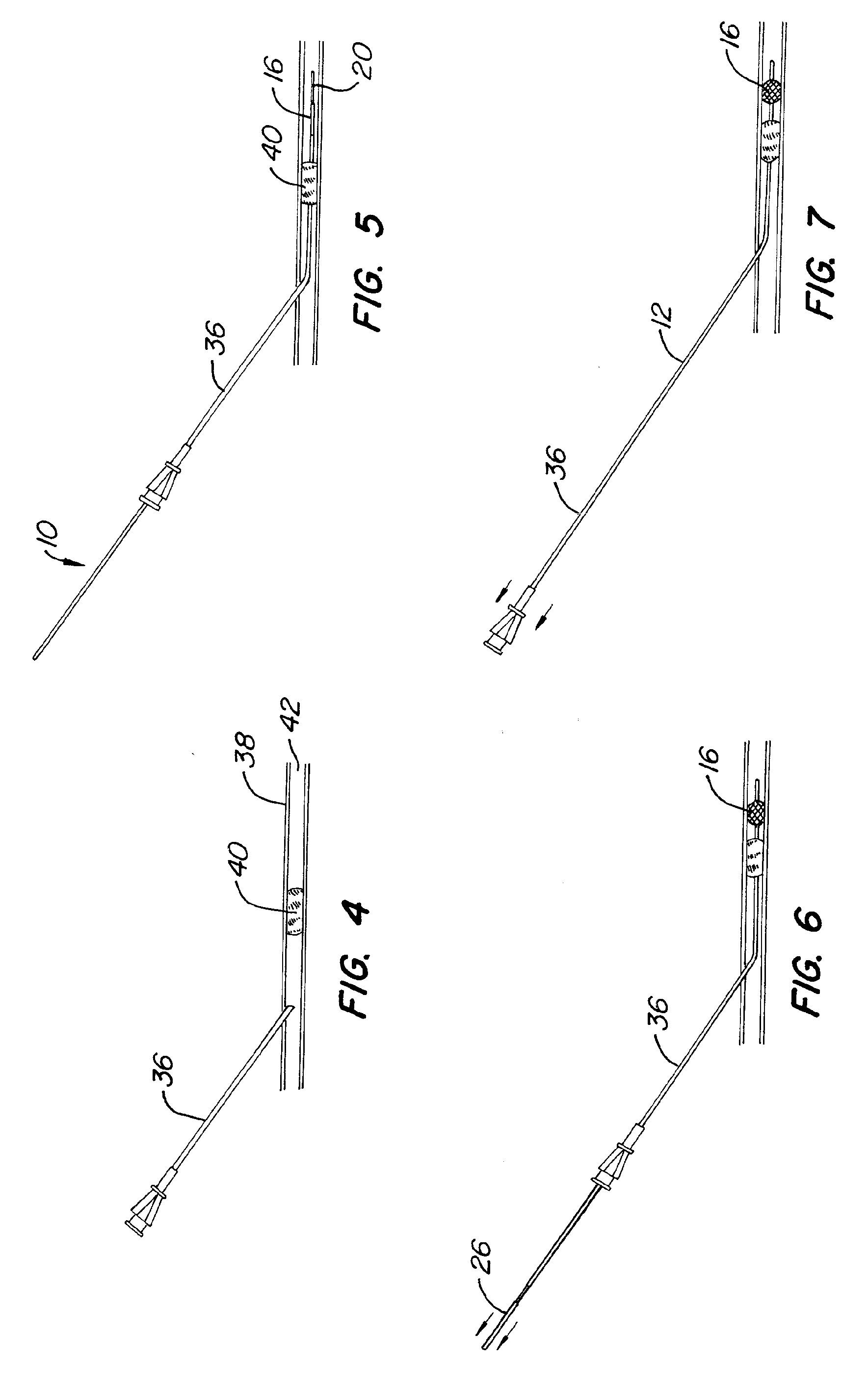
Medical Devices and Methods
a technology applied in the field of medical devices and methods, can solve the problems of difficult to reach the area to be treated, difficult to thread the needle of the arteries or other vessels, and difficult management of the guide wire in the operating room, and achieve the effect of small siz
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
Clot Dragger Lock
[0136]One aspect of the instant invention relates to a locking mechanism for the blocking or engaging element. Of particular relevance is the locking mechanism of the engaging element. One such preferred embodiment incorporates an interference fit when and inner and outer slidable elongate member is used. Once deployed, the force required to keep the engaging element is usually small in relation to the force required to deploy (in the case of a non-self-expanding mechanism). In this case, a slight interference fit between the inner and outer slidable elongate members can be overcome easily by the interventionalist, but when the engaging or blocking element is deployed (partially or fully), the interference fit creates enough force of the system to remained deployed. The same invention could be used in the case where either the engaging element or blocking element is self-expanding, but in this case the interference fit would keep either element in the un-deployed, u...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com