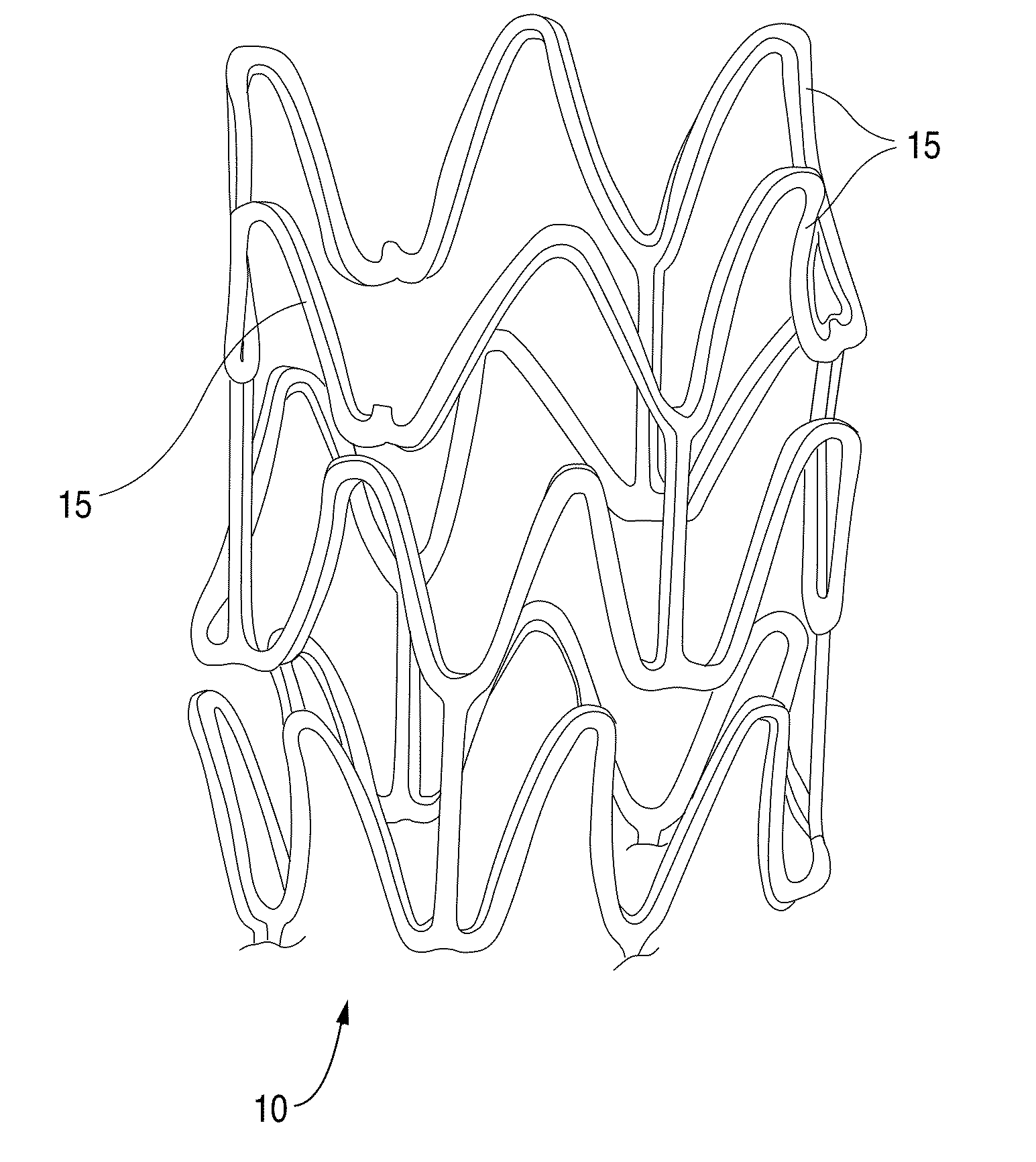
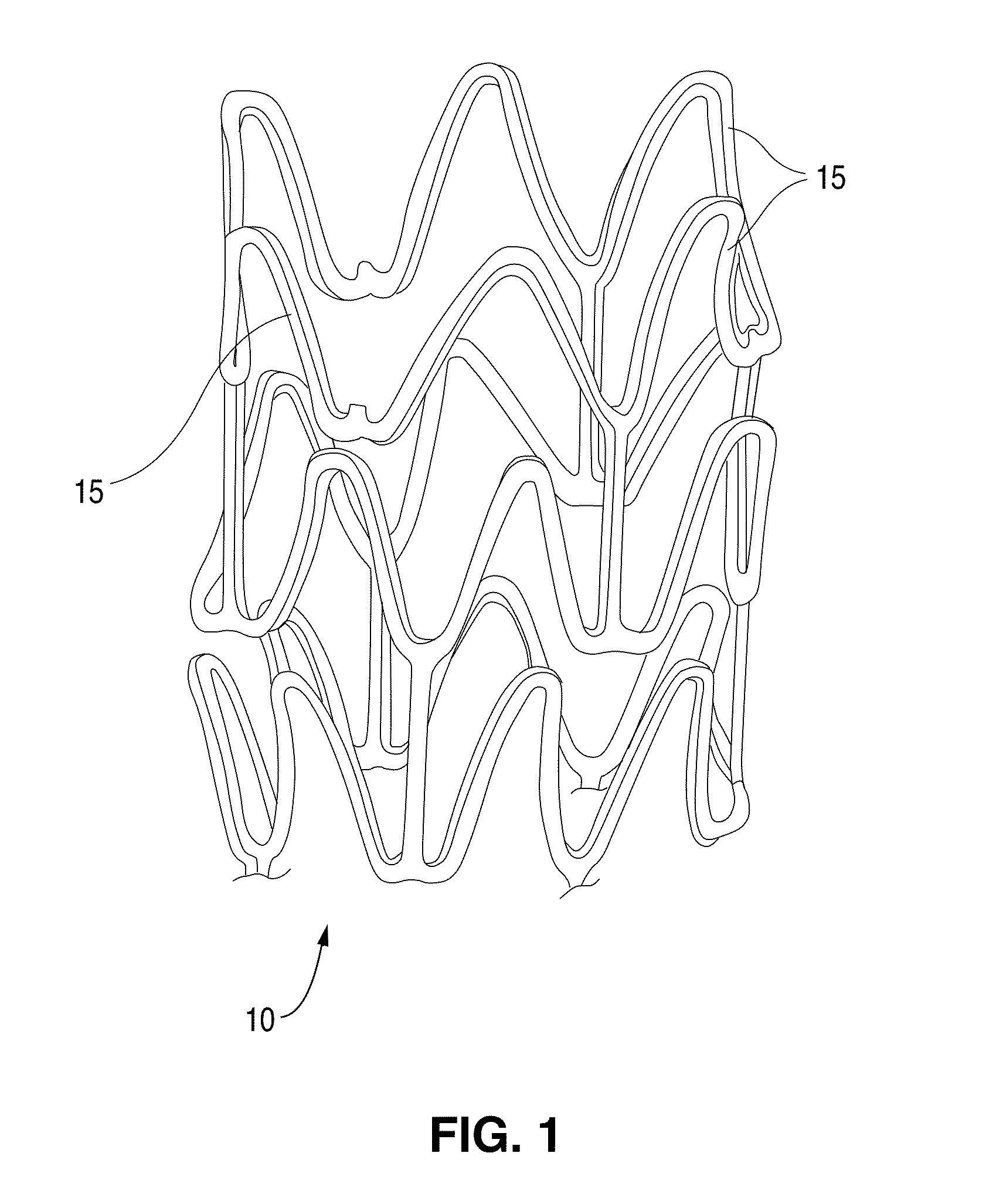
Methods Of Providing Antioxidants To Implantable Medical Devices
a technology of medical devices and antioxidants, applied in the field of chemical engineering and medical devices, can solve the problems of affecting the quality of life of patients, and prolonging the recovery period, so as to achieve the effect of reducing the risk of complications, avoiding surgery, and reducing the effect of surgery
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
Sublimation of BHT
[0110]A study was performed that demonstrated the sublimation of BHT. In the first experiment, 100 mg of BHT was weighed in an aluminum pan and heated in a convection oven at 55° C. At 1 hour and 16 hours after placement in the oven, the pan was removed and weighed. At the next time point, after leaving the pan in the oven overnight, no solids were present. A subsequent experiment was carried out utilizing a temperature of 70° C., and weighted at time-points of 30 minutes and 1 hr. The melting point of BHT is 70° C. so this temperature was the highest temperature in the experiments. Additional data was obtained from measurements at 40° C. and 50° C. with weight measurements at time-points of 1, 4, 7 and 24 hours. The results are illustrated in FIG. 2 which shows a plot of BHT weight loss vs. time for the different temperatures. As shown in FIG. 2, it is clear that BHT sublimation occurred at temperatures under 70° C.
[0111]FIG. 3 is a plot of Ln (BHT / BHT0) vs. time....
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com