Modafinil compositions
a technology of compositions and modafinil, which is applied in the direction of drug compositions, biocide, organic chemistry, etc., can solve the problems of filtration problems, non-uniform mixtures, and needle-like morphologies
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
2:1 R-(−)-modafinil:S-(+)-modafinil
[0146]Anhydrous ammonia gas was bubbled through a solution containing R-benzhydrylsulfinyl methyl ester (8.62 g, 0.0299 mol, about 80:20 R-isomer:S-isomer by weight) in methanol (125 mL) for 10 minutes. The pressure build-up from the reaction caused a back flow of sodium bicarbonate from the trap into the reaction mixture. The reaction was stopped and the precipitate was collected. The filtrate was concentrated under reduced pressure to give a yellow solid residue (2.8 g). The yellow solid was passed through a column (silica gel, grade 9385, 230-400, mesh 60 angstroms), 3:1 v / v ethyl acetate:hexane as eluent). The filtrates were then combined and concentrated under reduced pressure to give a slightly yellow solid (most of the yellow color remained on the column). The solid was then re-crystallized from ethanol by heating the mixture until it was boiling and then cooling to room temperature to give 2:1 R-(−)-modafinil:S-(+)-modafinil as a colorless ...
example 2
Polymorphs of R-(−)-modafinil
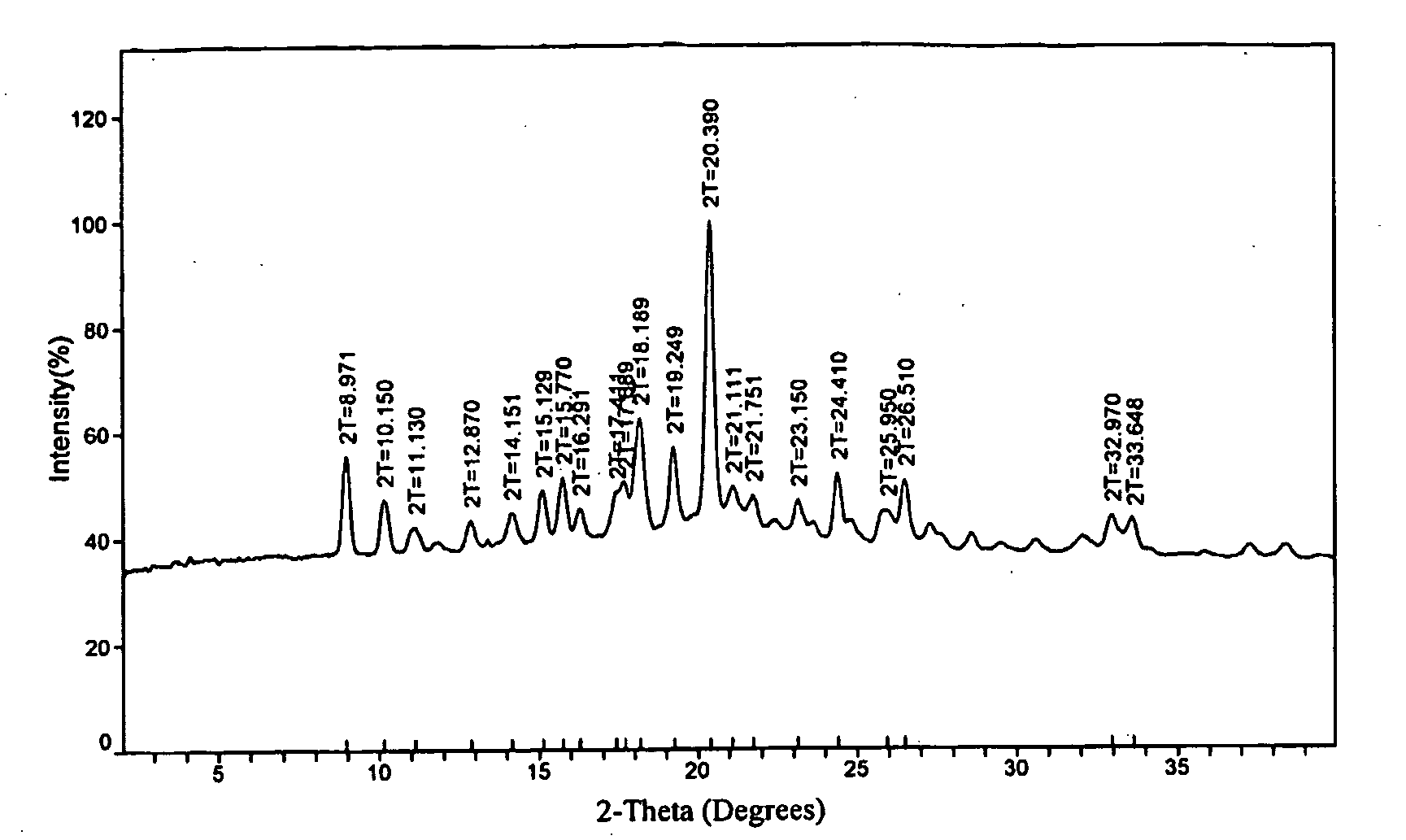
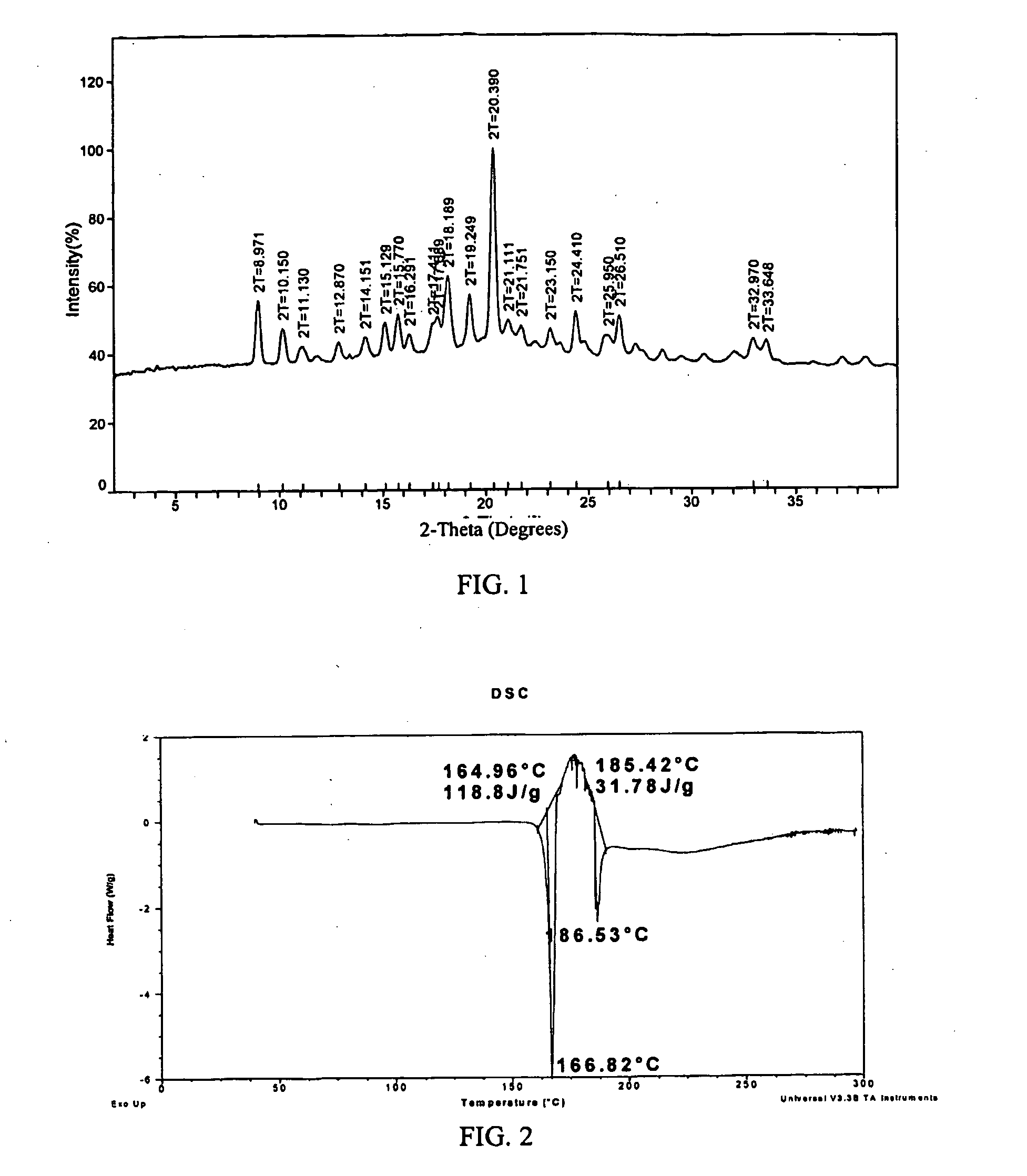
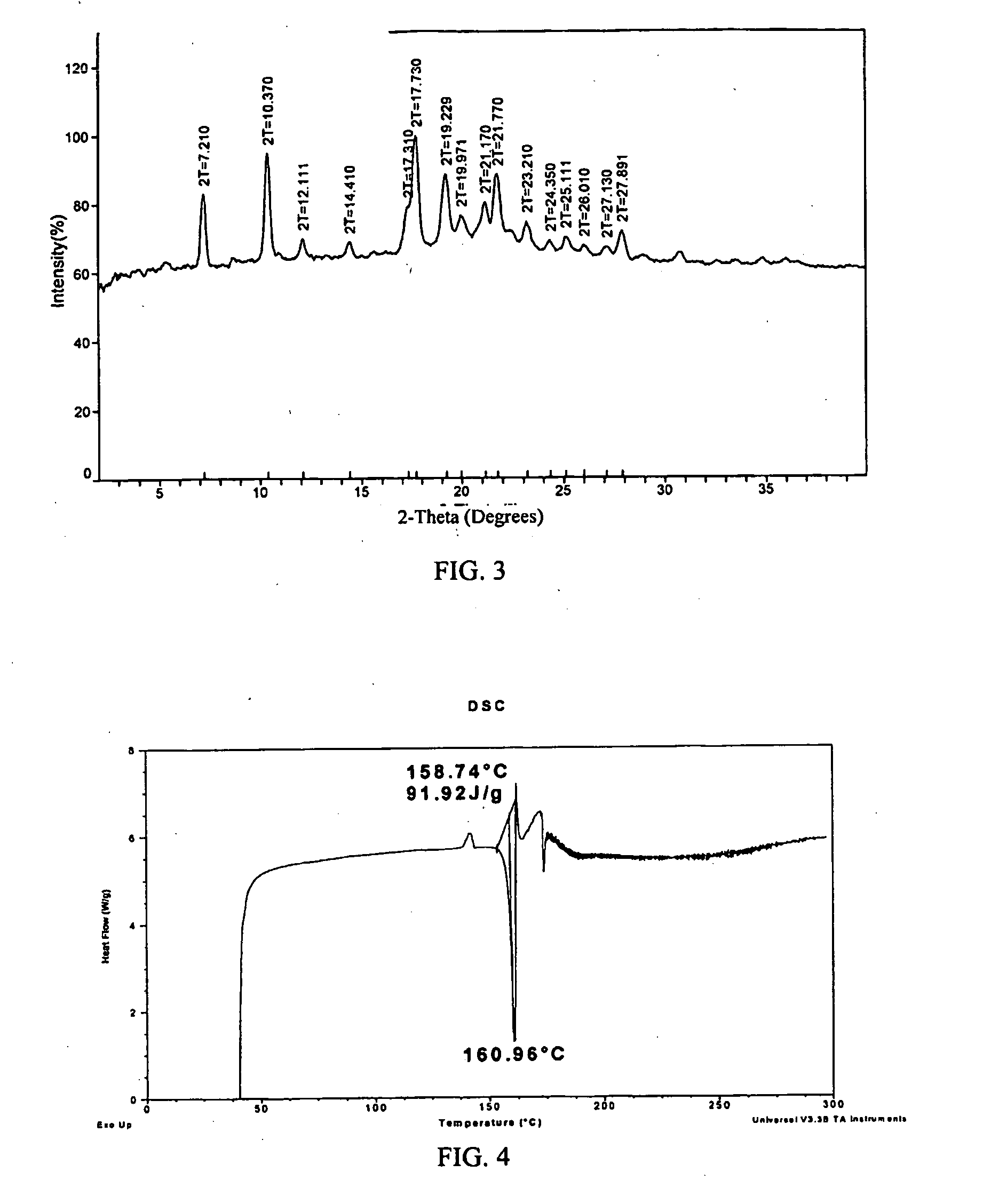
[0149]Several polymorphs of R-(−)-modafinil have been observed, each characterized by PXRD. FIGS. 3, 6, and 9 show these PXRD diffractograms (data as collected) of polymorphs Form III, Form IV, and Form V.
[0150]Recrystallization has proved to be an effective technique for the formation and acquisition of the polymorphs of R-(−)-modafinil. Suitable solvents for the crystallization of one or more polymorphs of R-(−)-modafinil include, but are not limited to, acetonitrile, dimethyl formamide (DMF), methanol, methyl ethyl ketone, N-methyl pyrollidone, ethanol, isopropanol, isobutanol, formamide, isobutyl acetate, 1,4-dioxane, tetrahydrofuran (THF), ethyl acetate, o-xylene, isopropyl acetate, dichloromethane, propylene glycol, acetic acid, water, acetone, nitromethane, toluene, and benzyl alcohol. Pure solvents and mixtures of solvents may be used to crystallize one or more polymorphs of R-(−)-modafinil.
R-(−)-modafinil Form III
example 3
2:1 R-(−)-modafinil:S-(+)-modafinil
[0172]A solution containing R-(−)-modafinil (80.16 mg, 0.293 mmol) and racemic modafinil (20.04 mg, 0.0366 mmol) in ethanol (2 mL) was prepared. The mixture was heated to boiling in order to dissolve the entire solid and was then cooled to room temperature (25 degrees C.). After remaining at room temperature for 15 minutes, the solution was placed at 5 degrees C. overnight. The solution was then decanted and the remaining crystals were dried under a flow of nitrogen gas and characterized using HPLC, PXRD, DSC, and thermal microscopy.
[0173]The crystals obtained contained between about 63 and about 67 percent R-(−)-modafinil and the remainder of the crystals was S-(+)-modafinil. HPLC analysis indicated that the crystals were a 2:1 phase containing two R-(−)-modafinil molecules for every one S-(+)-modafinil molecule.
[0174]PXRD was completed on a single crystal sample of the 2:1 R-(−)-modafinil:S-(+)-modafinil. 2:1 R-(−)-modafinil:S-(+)-modafinil can b...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Angle | aaaaa | aaaaa |
| Angle | aaaaa | aaaaa |
| Angle | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com