Medicament for treatment of non-insulin dependent diabetes mellitus, hypertension and/or metabolic syndrome
a non-insulin-dependent, metabolic syndrome technology, applied in the direction of biocide, plant ingredients, sugar derivatives, etc., can solve the problems of increasing the risk of cardiac arrhythmias, many unwanted adverse effects, and patients' hypertension and dyslipidemia, so as to suppress the total cholesterol level, suppress appetite, and suppress fasting plasma triglycerides
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
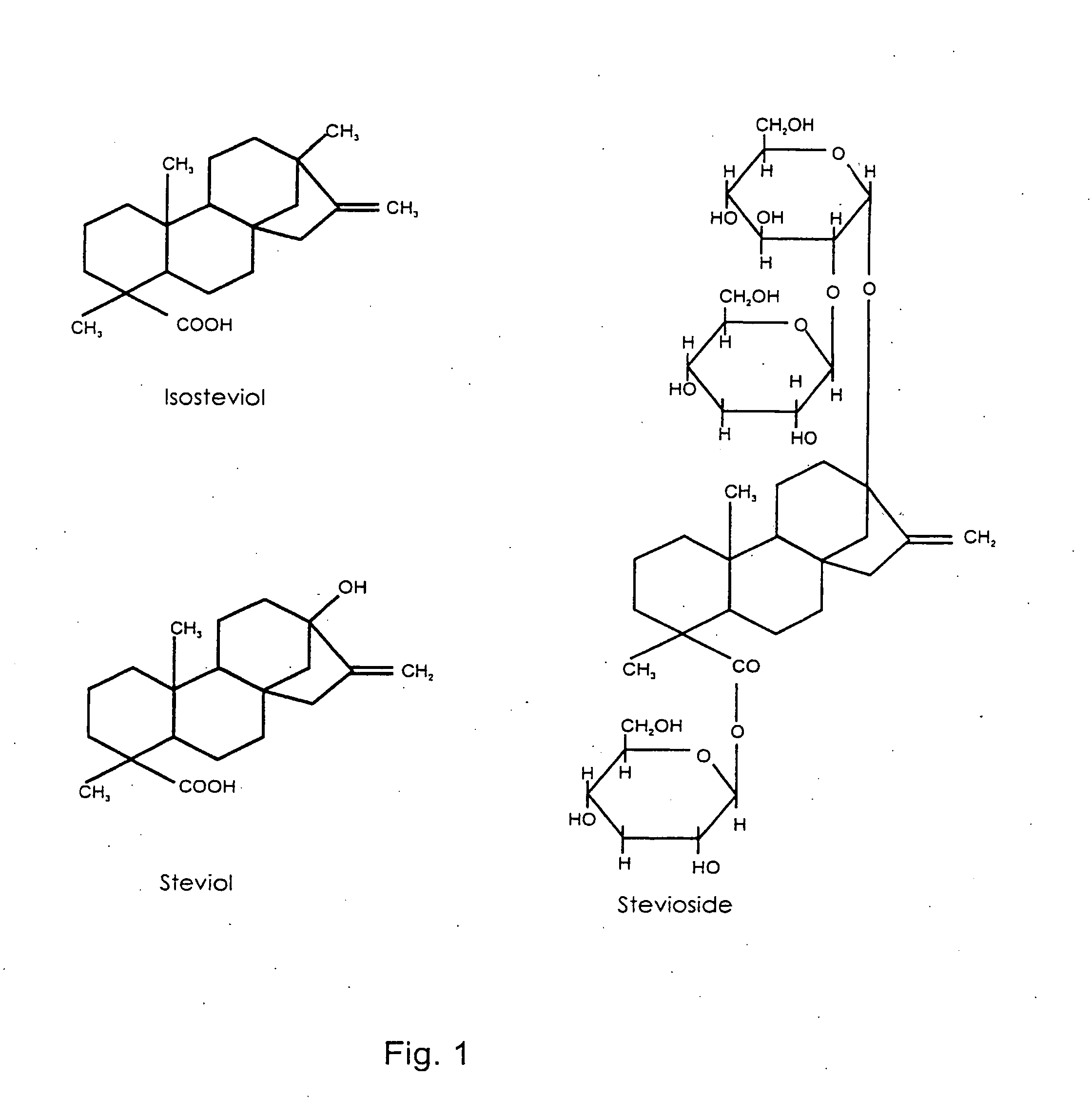
[0084] As examples of the effects of a compound including the chemical formulas II, stevioside was tested on normal Wistar rats and on GK rats. 2.0 g glucose / kg body weight and 0.2 g stevioside / kg body weight were dissolved in 0.9% saline and infused intravenously. The plasma glucose and insulin levels were measured over a period of 2 hours.
[0085] The results are shown in FIGS. 2a, 2b, 3a and 3b, were the O-O series (n=6 for Wistar and n=14 for GK) illustrate glucose infused alone and the ②-② series (n=6 for Wistar and n=12 for GK) illustrate the combined glucose and stevioside infusion. Data are given as mean ±SEM.
[0086] After administration of the glucose load, plasma glucose raised immediately and plasma insulin raised abruptly. When stevioside was added together with the glucose, a diminished glucose response was found in the GK-rat and a significant decrease was observed already after 30 min. In the GK rat, stevioside caused a pronounced increase in the insulin response compa...
example 2
[0088] Islet from 6-10 NMRI mice were isolated and incubated in the presence of 16.7 mmol / l and 10−9-10−3 mol / l stevioside or 10−9-10−3 mol / l steviol.
[0089] The results of these tests are illustrated in FIGS. 4a and 4b where each column represents mean ±SEM from 24 incubations of single islets. Black bars in FIG. 4a indicate that stevioside is present and hatched bars indicate that stevioside is absent.
[0090] Black bars in FIG. 4b indicate that steviol is present and hatched bars indicate that steviol is absent.
[0091] The figures show that stevioside and steviol are capable of potentiating glucose-stimulated insulin secretion. Further tests confirmed that a stimulatory effect was found already at a very low concentration (above 0.1 nM).
example 3
[0092] During a glucose tolerance test, an intravenous bolus of stevioside of 0.2 g / kg body weight was injected in GK rats (the ②-② series (n=6)). GK rats receiving 0.9% saline intravenously served as controls (the O-O series (n=6)). Glucose 2.0 g / kg body weight was administered as a bolus at timepoint 0 min. The plasma glucagon responses are shown as mean ±SEM in FIGS. 5a (control) and 5b (GK). The plasma glucagon was suppressed in the stevioside treated GK rat.
PUM
| Property | Measurement | Unit |
|---|---|---|
| weight | aaaaa | aaaaa |
| weight | aaaaa | aaaaa |
| body weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com