Antimicrobial conjugates
a technology of conjugates and antimicrobials, applied in the field of antimicrobial conjugates, can solve the problems of inherently unstable nanoparticle suspensions, the ability of nanoparticles to kill either mammalian cells or microorganisms, etc., and achieve the effect of killing or inhibiting growth and demonstrating antimicrobial activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
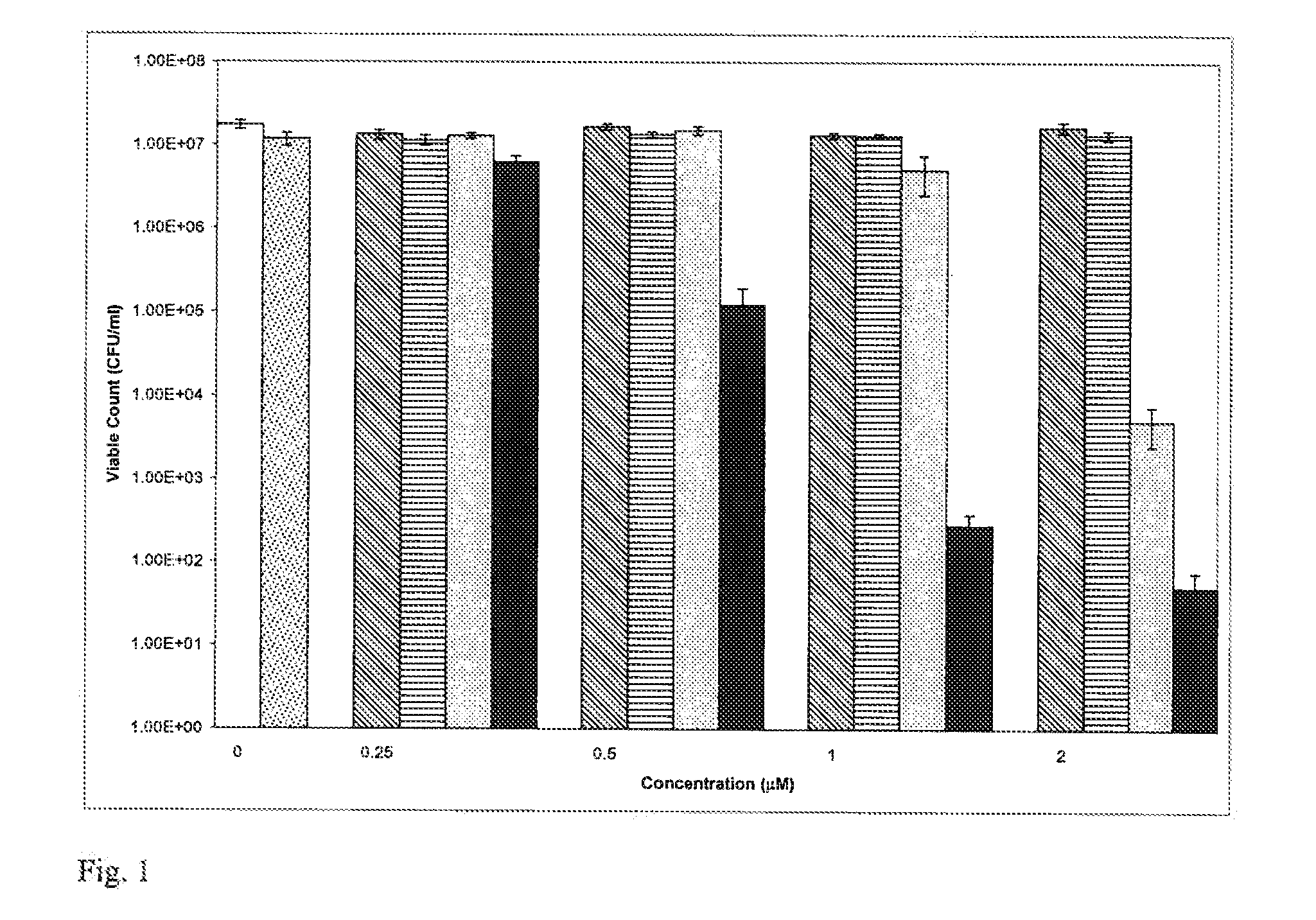
[0151] Gold nanoparticles (2.0 nm diameter; British Biocell International) in water (15×1013 particles per ml) were mixed with an equal volume of an aqueous solution of toluidine blue O (40 μM) and left at room temperature for 15 minutes. 100 μl of the gold-TB solution was added to 100 μl of a suspension of Staphylococcus aureus NCTC 6571 in phosphate buffered saline (PBS) and this was irradiated with white light from a fluorescent white lamp for 10 minutes. Controls consisted of:
[0152] (i) TB (final concentration=10 μM) and bacteria, irradiated for the same period of time,
[0153] (ii) nanogold (diluted 1:1 with water) and bacteria, irradiated for the same period of time,
[0154] (iii) bacteria without TB or nanogold, not irradiated (“control”).
[0155] After irradiation, the number of surviving bacteria was determined by viable counting. The results of the experiments (carried out twice with duplicate counts on each occasion) are shown in Table 1. The gold nanoparticles alone when i...
example 2
Production of Water-Soluble Gold Nanoparticles
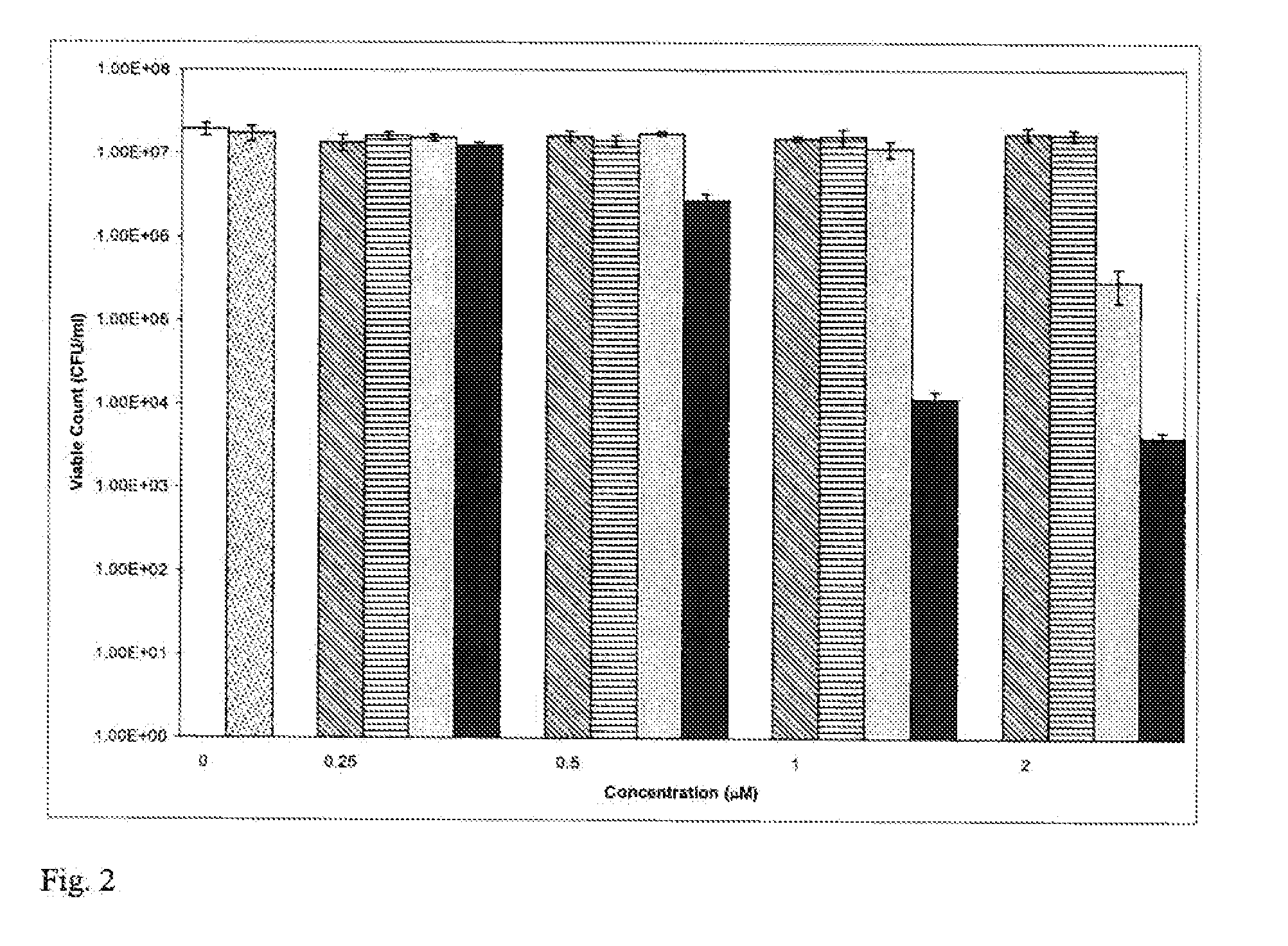
[0156] HAuCl4.3H2O (42 mg, 0.11 mmol) was dissolved in deionised water (25 ml) to form solution A (˜5 mM). Na3C6H5O7.2H2O (125 mg, 0.43 mmol) was dissolved in deionised water (25 ml) to give solution B (˜20 mM). Solution A (1 ml) was stirred with deionised water (18 ml) and boiled for 2 min. Then solution B (1 ml) was added dropwise over a period of approximately 50 sec. causing the colour change from clear to blue to pink / purple. After a further 1 min. of heating, the solution was left to cool to room temperature. Two batches of nanogold particles were used for subsequent antibacterial assays—these are designated NN1 and NN2. The absorption spectrum of NN2 showed the wavelength of maximum absorption, λmax to be 527 nm. Batch NN1 had a λmax of 522 nm. Particle size analysis (position of UV plasmon absorption band measured using transmission electron microscope) of batch NN1 gave an average diameter of 14.76±2.34 nm.
Effect of Concentr...
example 3
[0159] The method of Example 2 was repeated using gold nanoparticles of 2 nm diameter (British Biocell International). The final concentration of nanogold used was 4×1013 particles / ml. TB was used at a final concentration of 10, 20 or 50 μM. The results are shown in Table 2 below. When the 2 nm nanogold particles were used, enhancement of lethal photosensitisation was evident using 20 μM TB but not when either 10 μM or 50 μM TB was used.
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com