Particles for inhalation having sustained release properties
a technology of inhalation and particle, which is applied in the direction of peptide/protein ingredients, inorganic non-active ingredients, metabolic disorders, etc., can solve the problem of rapid release of agents, and achieve the effect of reducing the initial release of agents that are typically seen in inhalation therapy, increasing the amount of time, and increasing patient compliance and comfor
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0027]A description of preferred embodiments of the invention follows.
[0028]Therapeutic, prophylactic or diagnostic agents, can also be referred to herein as “bioactive agents,”“medicaments” or “drugs.”
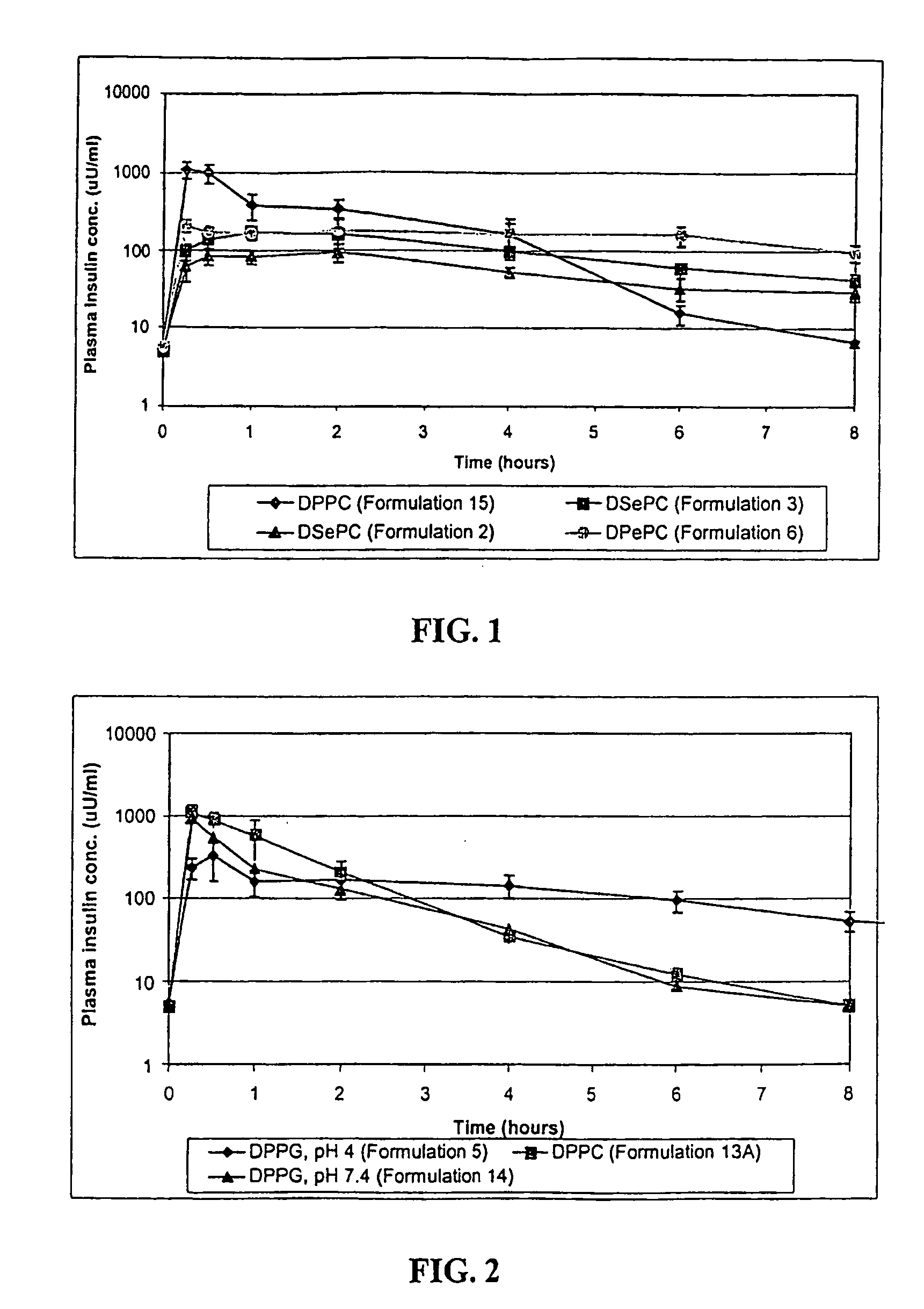
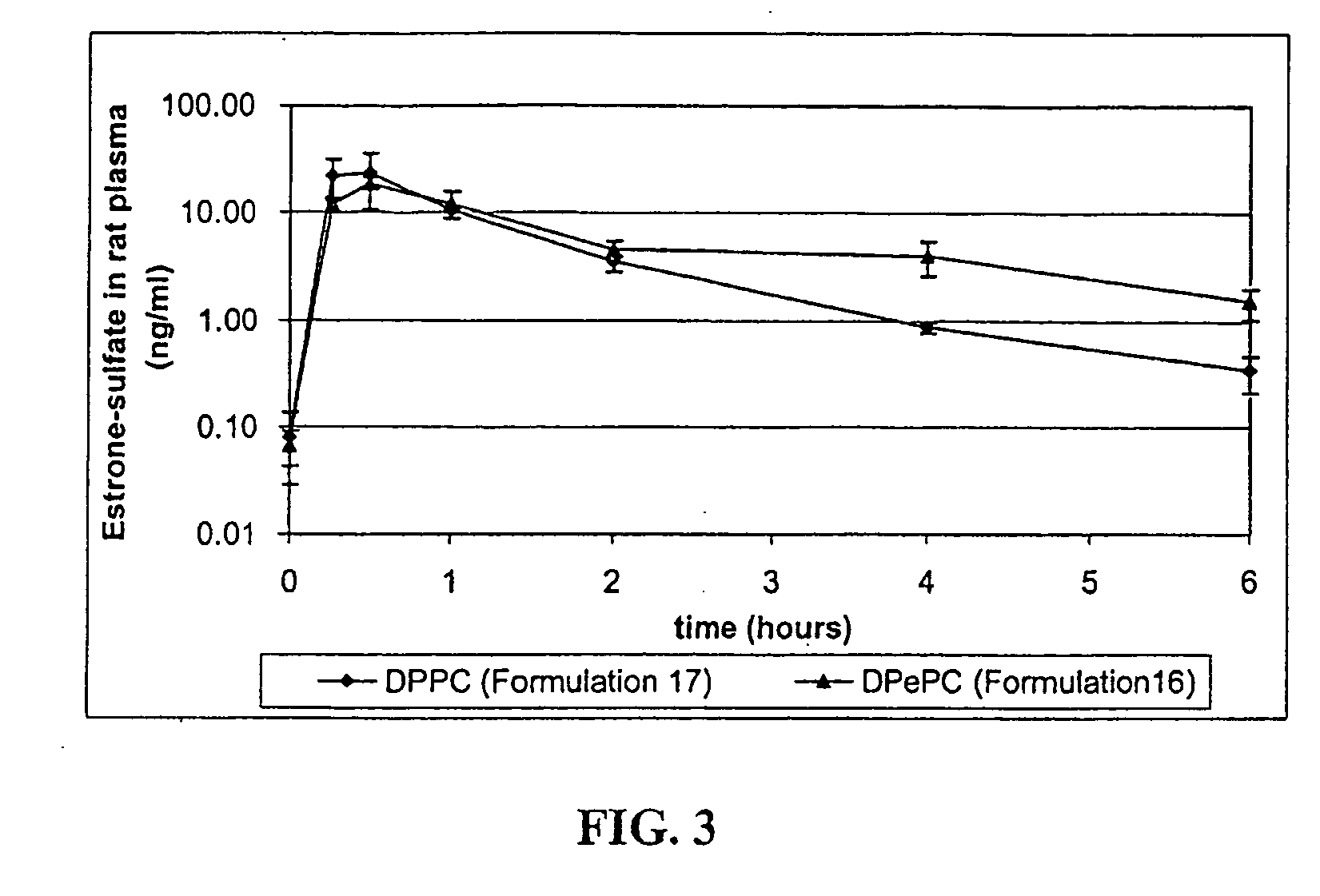
[0029]The invention relates to a method for the pulmonary delivery of therapeutic, prophylactic and diagnostic agents comprising administering to the respiratory tract of a patient in need of treatment, prophylaxis or diagnosis an effective amount of particles comprising a therapeutic, prophylactic or diagnostic agent or any combination thereof in association with a charged lipid, wherein the charged lipid has an overall net charge which is opposite to that of the agent. The agent is released from the administered particles in a sustained fashion.
[0030]The particles of the invention release bioactive agent in a sustained fashion. As such, the particles possess sustained release properties. “Sustained release,” as that term is used herein, refers to a release of active agent in which t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| tap density | aaaaa | aaaaa |
| tap density | aaaaa | aaaaa |
| median geometric diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com