Inflatable device for use in surgical protocol relating to fixation of bone
Inflatable balloon devices compress cancellous bone to create a stable cavity, addressing the issue of bone fractures and collapse by restoring cortical bone position and enhancing bone strength, thus improving treatment outcomes for conditions like osteoporosis and avascular necrosis.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
I. Balloons for Anatomical Structures
[0083] A. Balloons for Vertebral Bodies
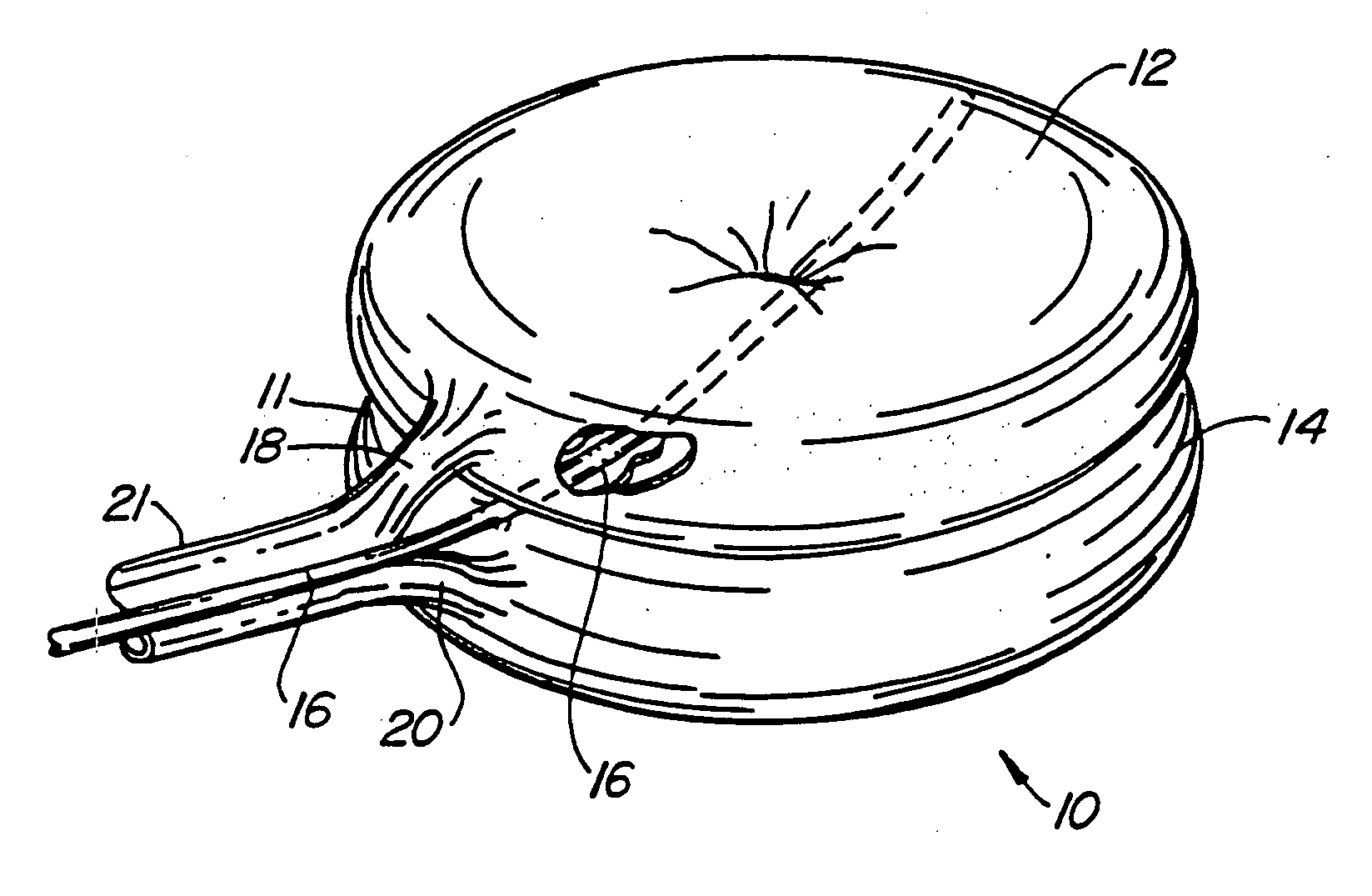
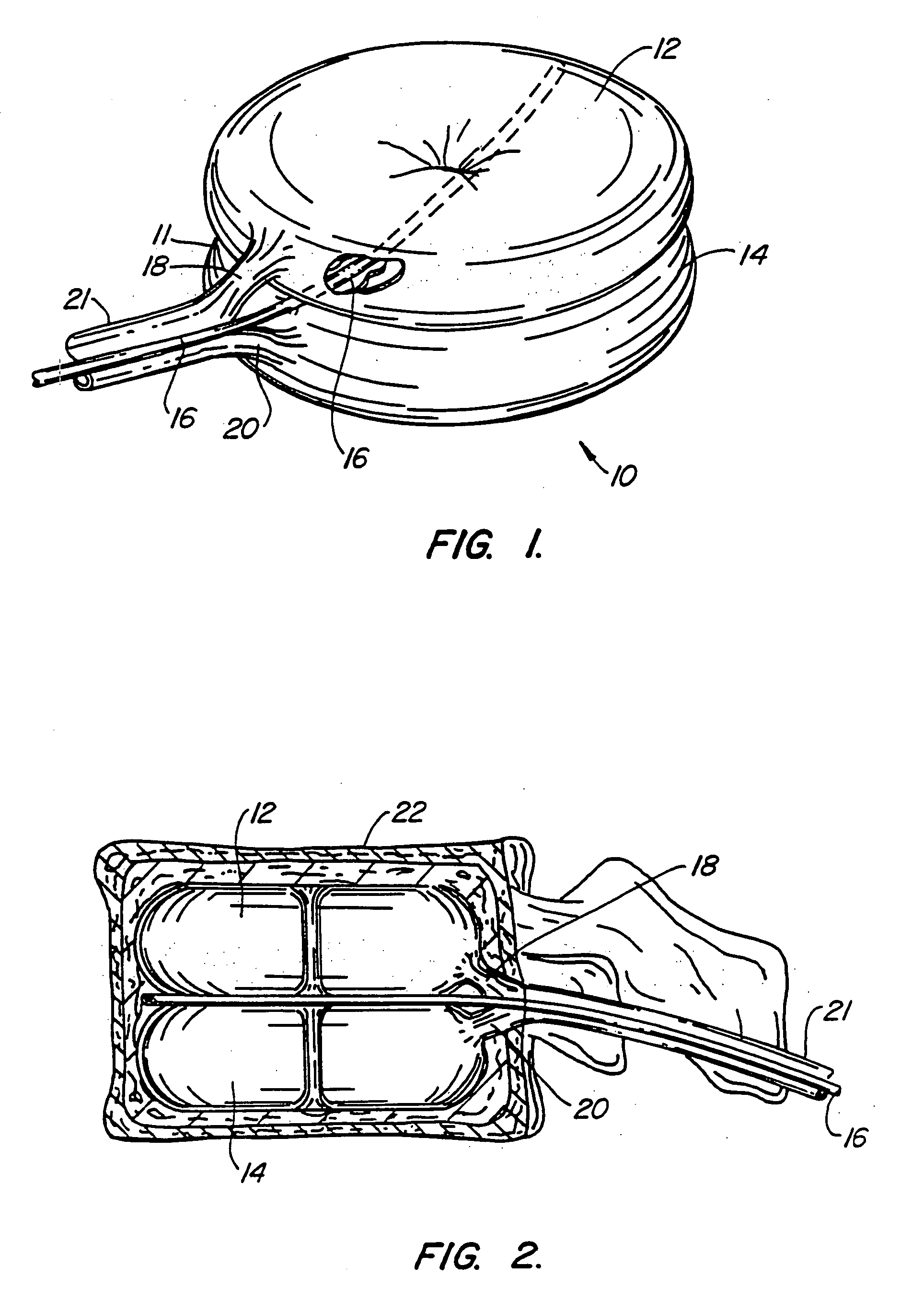
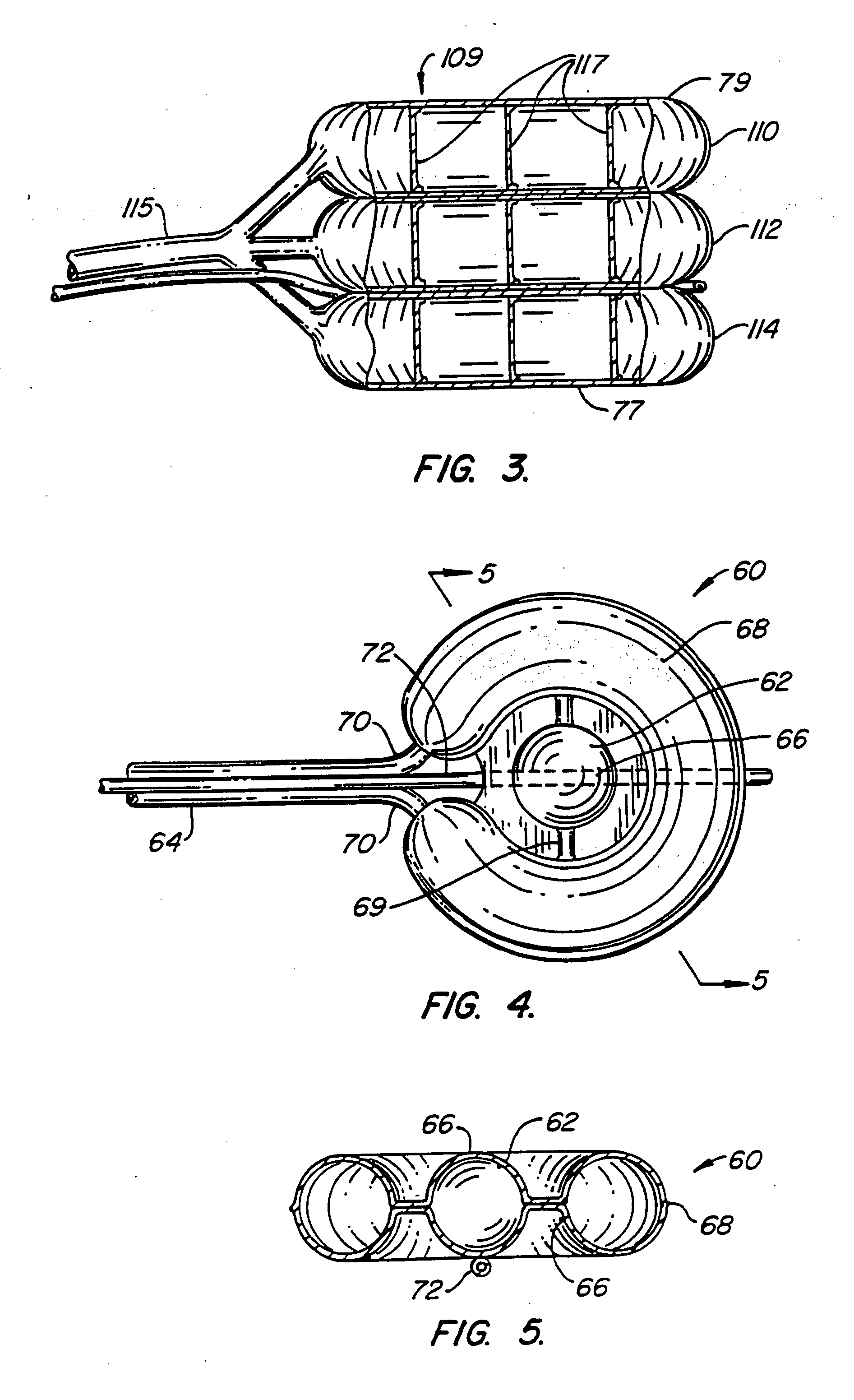
[0084] A first embodiment of the balloon (FIG. 1) constructed in accordance with the teachings of the present invention is broadly denoted by the numeral 10 and includes a balloon body 11 having a pair of hollow, inflatable parts 12 and 14 comprised of flexible material, including (but not limited to) non-elastic materials such as PET, mylar or Kevlar®, elastic materials such as polyurethane, latex or rubber, semi-elastic materials such as silicone, or other materials. Parts 12 and 14 have a suction tube 16 therebetween for drawing fats and other debris by suction into tube 16 for transfer to a remote disposal location. Tube 16 has one or more suction holes so that suction may be applied to the open end of tube 16 from a suction source (not shown).
[0085] In this embodiment, the parts 12 and 14 are connected together by an adhesive which can be of any suitable type for adhering such materials as well as by ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com